Initial attempts at minimally invasive esophagectomy (MIE) were hybrid operations combining traditional open surgery with minimally invasive techniques. The first report by Collard in 1993 included 6 patients who underwent thoracoscopic mobilization of the esophagus followed by laparotomy and preparation of the gastric conduit [1]. A completely laparoscopic transhiatal esophagectomy was also described by DePaula in 1995 [2], who described 12 patients requiring esophagectomy, predominantly for benign disease (end-stage achalasia secondary to Chagas disease). The first experience with MIE in North America was not reported until 1997, when Swanstrom described a group of 9 patients with small tumors, benign strictures, and Barrett’s disease [3]. Eight of these patients had a totally laparoscopic trans-hiatal esophagectomy, while one required the addition of a right video-assisted thoracoscopic surgery (VATS) procedure.
Since the first report, there has been a steady but slow rise in the number of institutions around the world performing this complex operation. There are several reasons for the rather slow pace of acceptance of this operation as the standard of care for esophageal resection. First, the adequacy of minimally invasive esophagectomy as an oncologically-sound operation has been challenged. Secondly, cost and utility of operating room time has been an issue. Finally, the low allocation of cases per surgeon impairs the surgeon’s ability to develop and refine the surgical technique and thus establish a “comfort level”.
We believe that the technically demanding nature and inherent complexity of this operation have been the main impediments to most thoracic surgeons accepting this operation. There are no large- scale randomized data to show superiority of outcome, but there are now several studies showing comparability of minimally invasive esophagectomy outcome with the open approach.
Francesco Palazzo et al (2015) undertook a study comparing minimally invasive esophagectomy (MIE) with open or hybrid esophagectomy (OHE) for patients with cancers of the esophagus and gastroesophageal junction [4]. The series contained 104 patients who underwent MIE and 68 patients who underwent open 3-hole, Ivor Lewis, or hybrid technique esophagectomy. Palazzo concluded that their results support MIE for esophageal cancer as a superior procedure with respect to five-year survival (MIE 64%, OHE 35%, p < 0.001), perioperative mortality (MIE 3.9% vs. OHE 8.8%, p = 0.19), and severity of postoperative complications.
Another study was undertaken by Fanyu Meng et al (2014) comparing the outcomes of open and minimally invasive esophagectomy in 183 patients with esophageal cancer [5]. In the study, 89 patients underwent open esophagectomy (OE) and 94 underwent thoracoscopic and laparoscopic esophagectomy (TLE). Their results showed the TLE method having significant advantages over OE with respect to blood loss (182.6±78.3 vs. 261.4±87.2 mL, P < 0.0001), hospital stay (13.9±7.5 vs. 17.1±10.2 days, P=0.017), surgical morbidity (25.5% vs. 46.1%, P=0.004), and rate of pulmonary and cardiac complication (9.6% vs. 27.0%, P=0.002; 4.1% vs. 12.4%, P=0.046). However, the paper reports no difference in survival period between the groups.
In a prospective comparison, Noble et al (2013) discuss the results of totally minimally invasive versus open Ivor Lewis esophagectomy [6]. The study had 106 patients who underwent esophagectomy, 53 of which were totally minimally invasive thoracolaparoscopic 2 stage esophagectomy (MIE-2). The study reported no significant difference in outcomes between the two approaches, aside from significantly less blood loss in MIE-2 cases (median 300 [range 0– 1250] ml vs. 400 [range 0–3000] ml, p = 0.021).
Urs Zingg et al (2009) also compared minimally invasive with open esophagectomy for patients with esophageal cancer [7]. The study reported outcomes in 56 (36%) attempted MIE cases and 98 (64%) open esophagectomy cases. Of the 56 attempted MIE procedures, 30 patients (53.6%) underwent a combined laparoscopic and thoracoscopic procedure and 26 (46.4%) underwent a thoracoscopically assisted esophagectomy. The results showed no significant differences between the groups and concluded that MIE and OE have comparable morbidity, mortality, and overall survival.
In a systematic review of the literature, Verhage et al (2009) compare minimally invasive to open procedures in esophagectomy for cancer [8]. The study selected 10 articles between 2000 and 2009 that compare open esophagectomy to some form of MIE, all of which were case-controlled studies with varying levels of evidence. Overall, the study compares 494 patients that underwent open transhiatal or transthoracic surgery, versus 616 patients receiving some form of MIE. The study concludes that the collective results point towards improved short term outcomes after MIE. Blood loss and hospital length of stay were significantly reduced in MIE cases. The study also found that morbidity and mortality rates were lower in minimally invasive approaches, with total complications being lower in most and comparable in some.
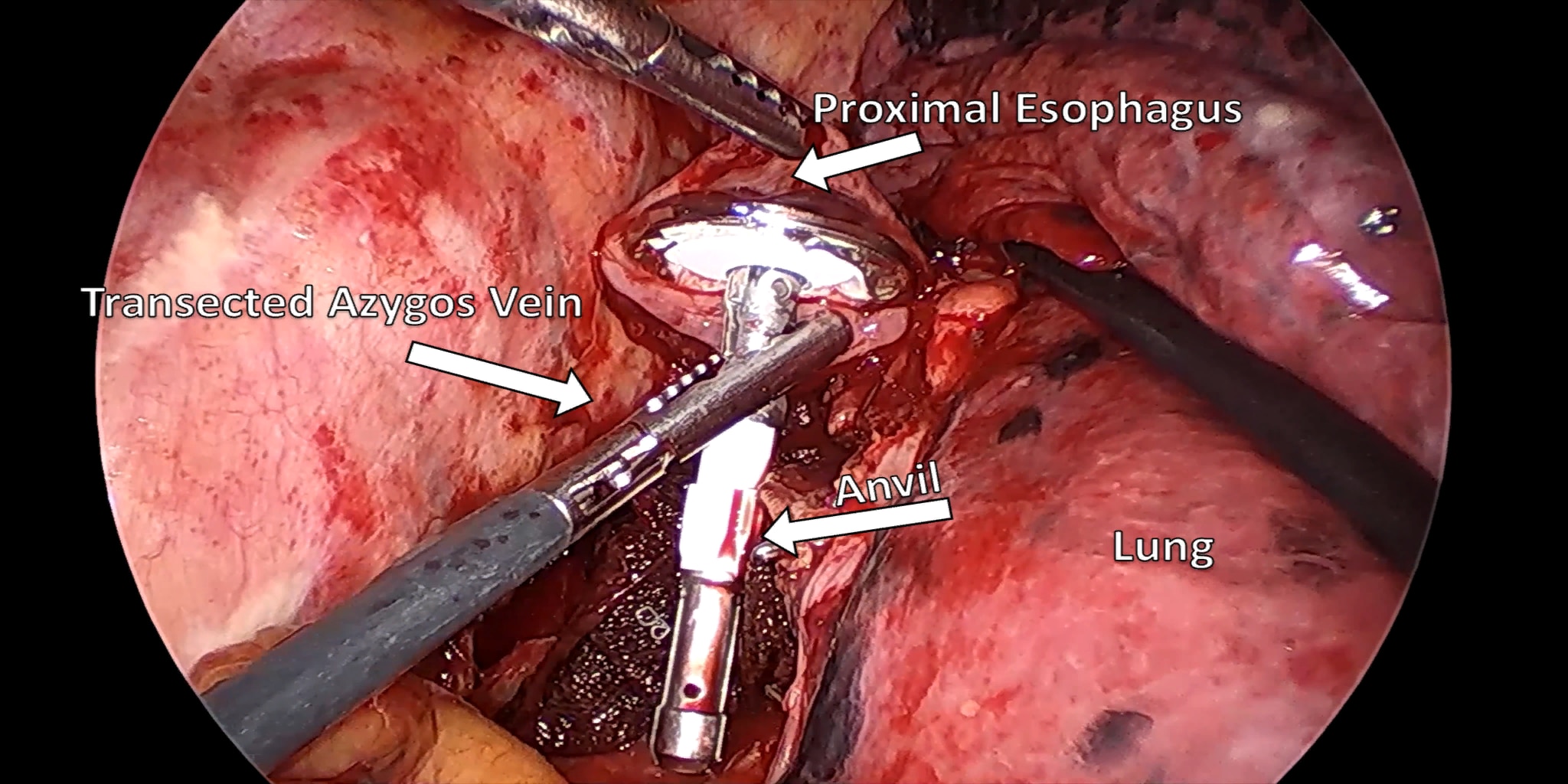
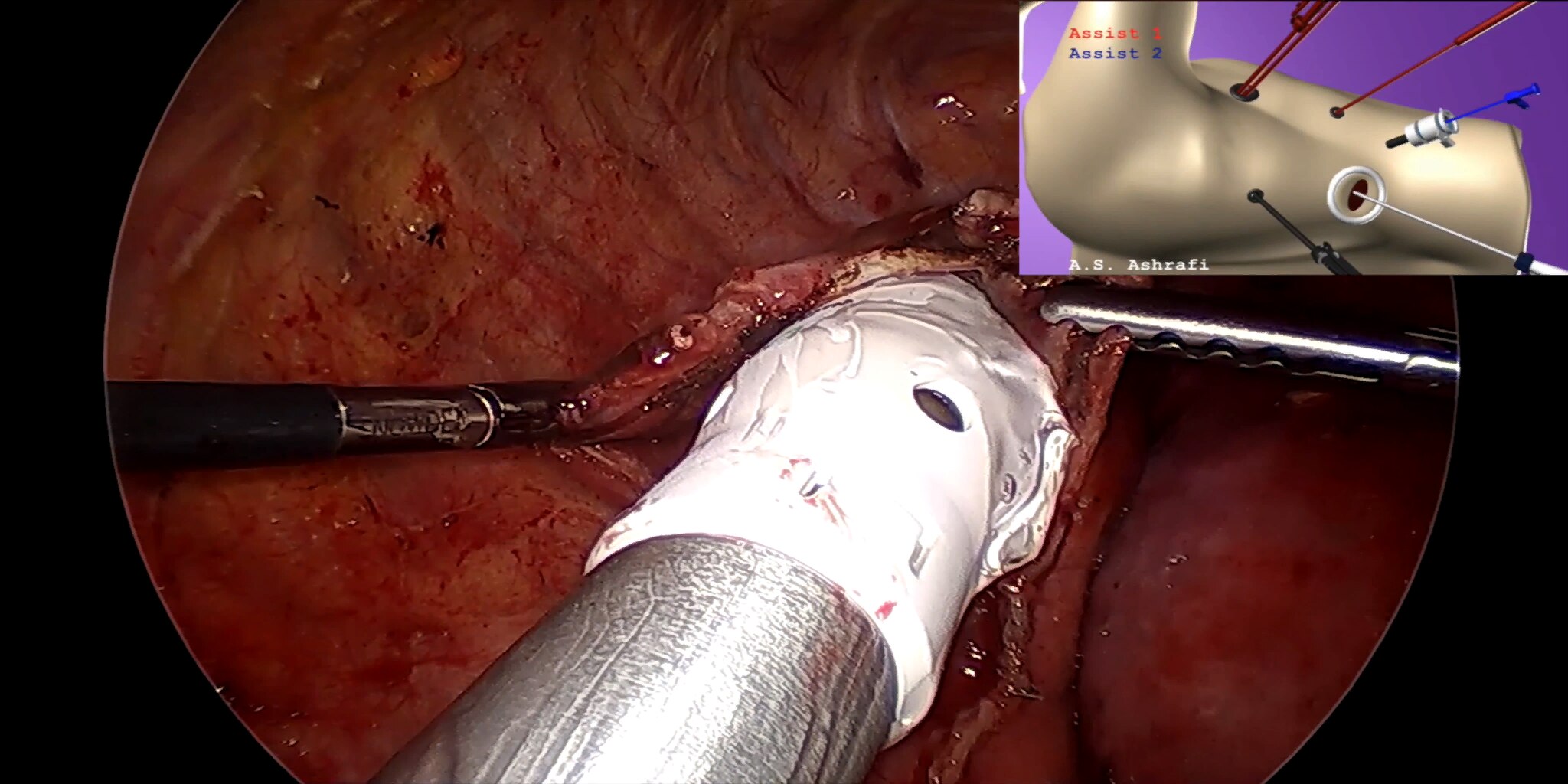
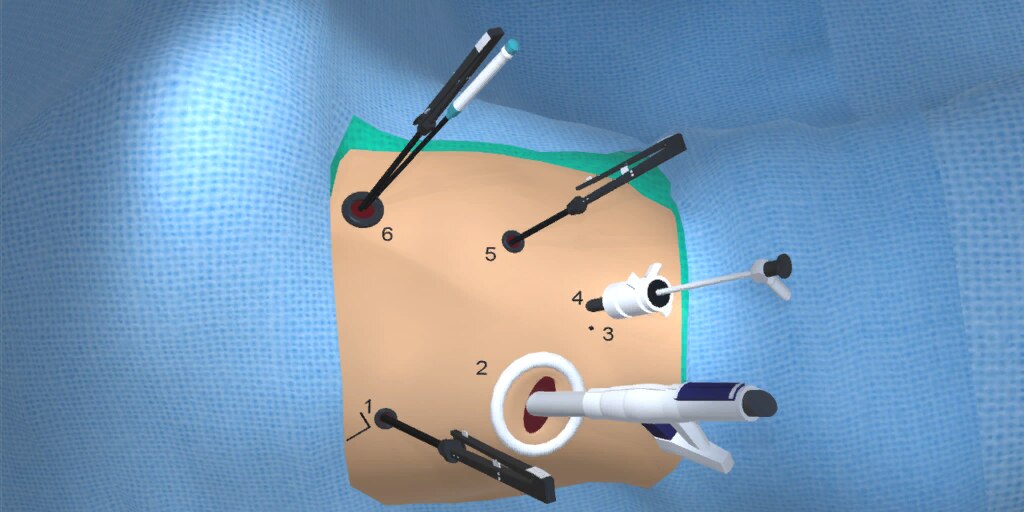
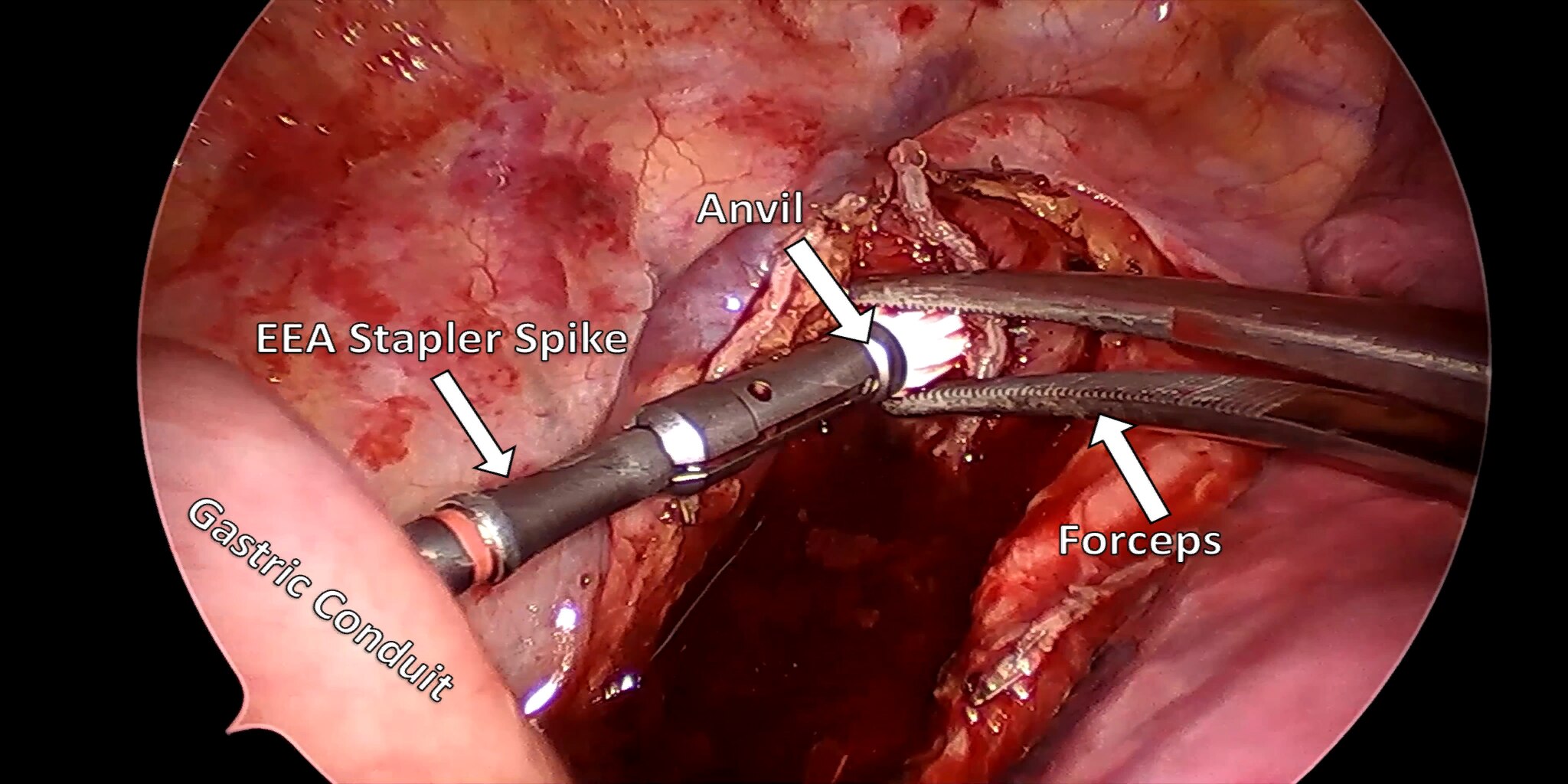
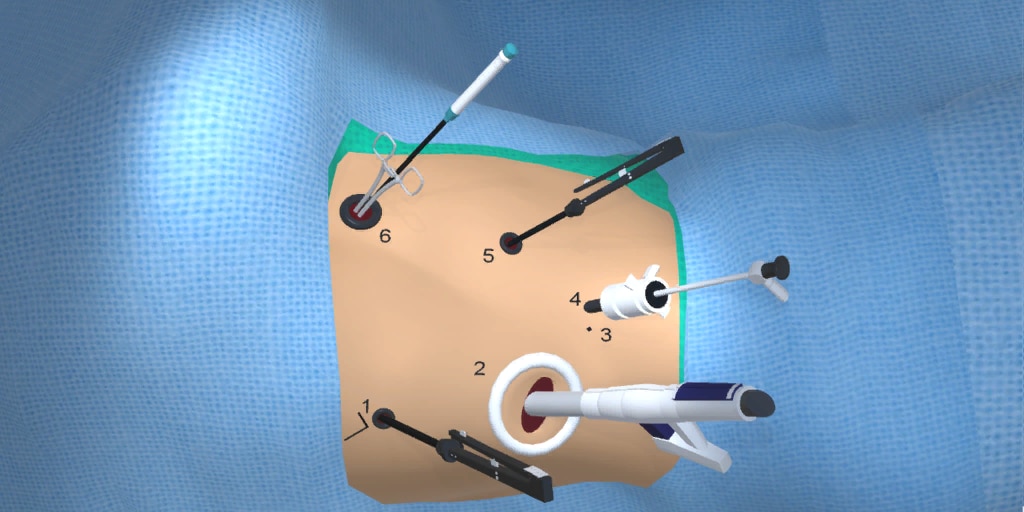
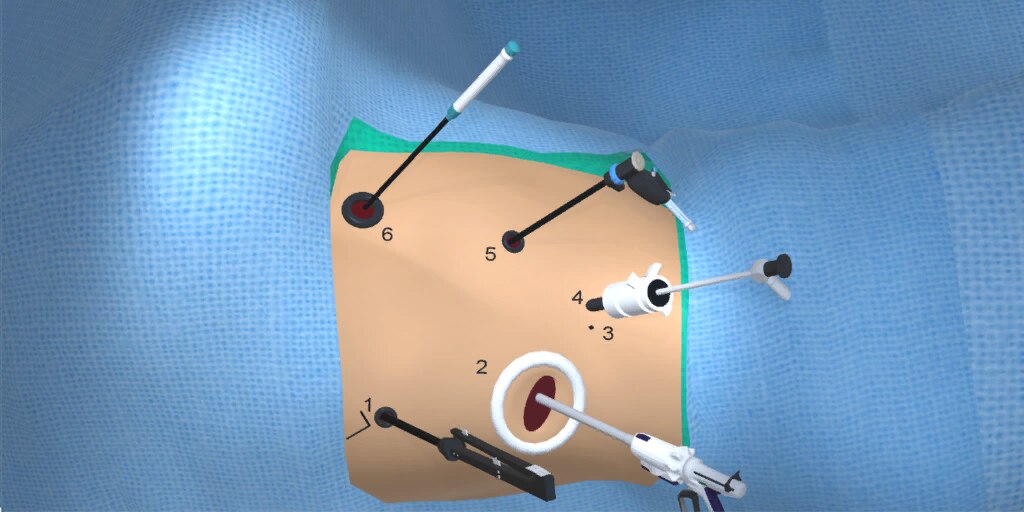
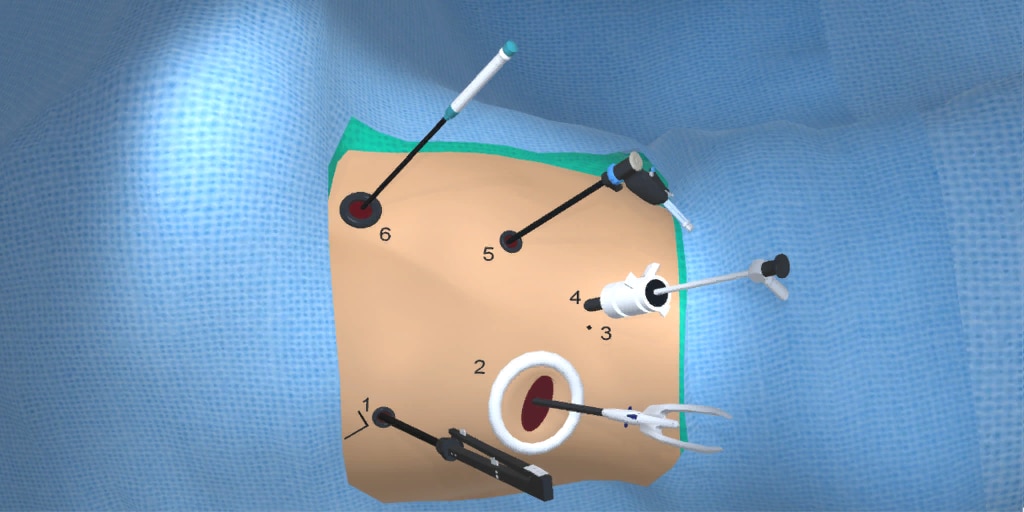
There are several minimally invasive techniques described for resecting the esophagus and the associated reconstruction: These include the transhiatal (laparoscopy and neck incision), the modified McKeown (Right VATS, laparoscopy, and neck incision), and the Ivor-Lewis (Laparoscopy and Right VATS with an intra-thoracic anastomosis). The prime objective of this paper is to provide the thoracic surgeon with appropriate pearls for mastering the minimally invasive Ivor-Lewis esophagectomy technique.
Ever since the report of the CROSS trial [9] esophagectomy as primary therapy for esophageal cancer has become less common, and trimodality therapy (chemoradiation followed by esophagectomy) has become the norm at most major centres around the world. The reported median overall survival was 49.4 months in the chemoradiotherapy-surgery group versus 24.0 months in the surgery group. Performing esophagectomy after chemoradiation does add additional complexity; however this is not a contra-indication and in fact, the significant majority of patients coming to esophagectomy for cancer nowadays receive induction chemoradiotherapy.
In an attempt to focus primarily on the technical aspects of minimally invasive Ivor-Lewis esophagectomy, the reader is encouraged to consult other sources for a discussion on preoperative preparation and work up of the patient prior to surgery, and for a discussion on the management of post-operative adverse outcomes. There will also be no discussion of outcomes of minimally invasive Ivor-Lewis esophagectomy.
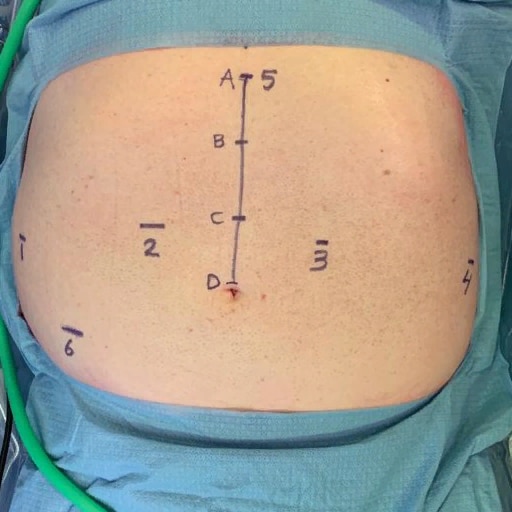
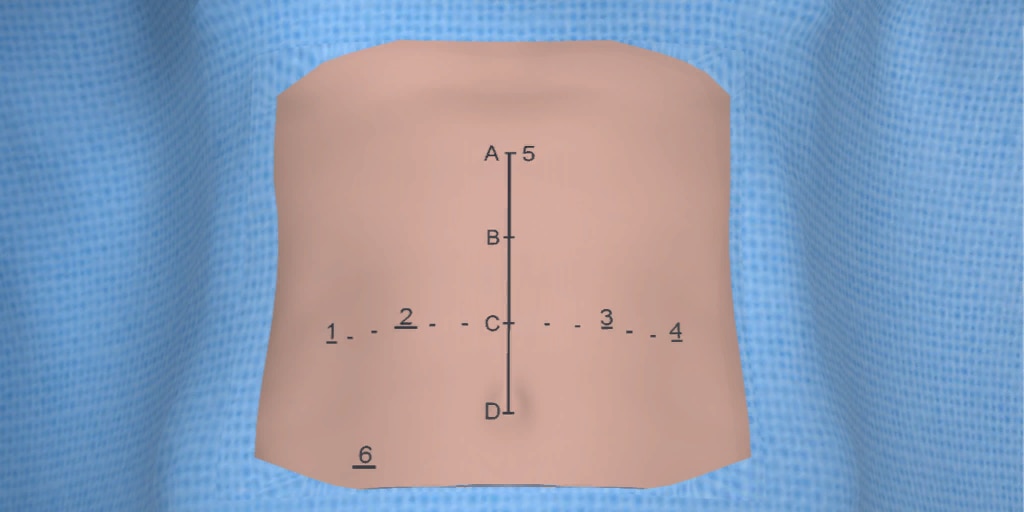
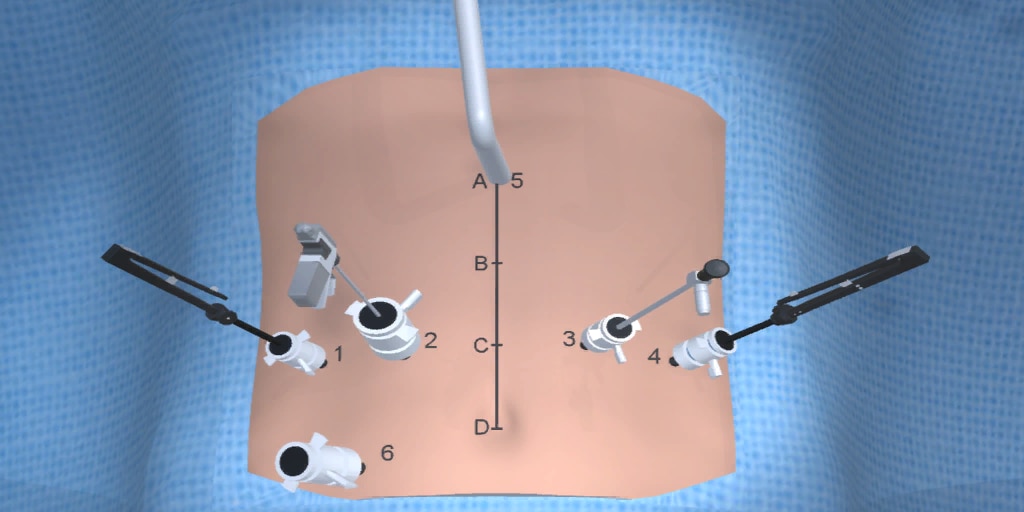
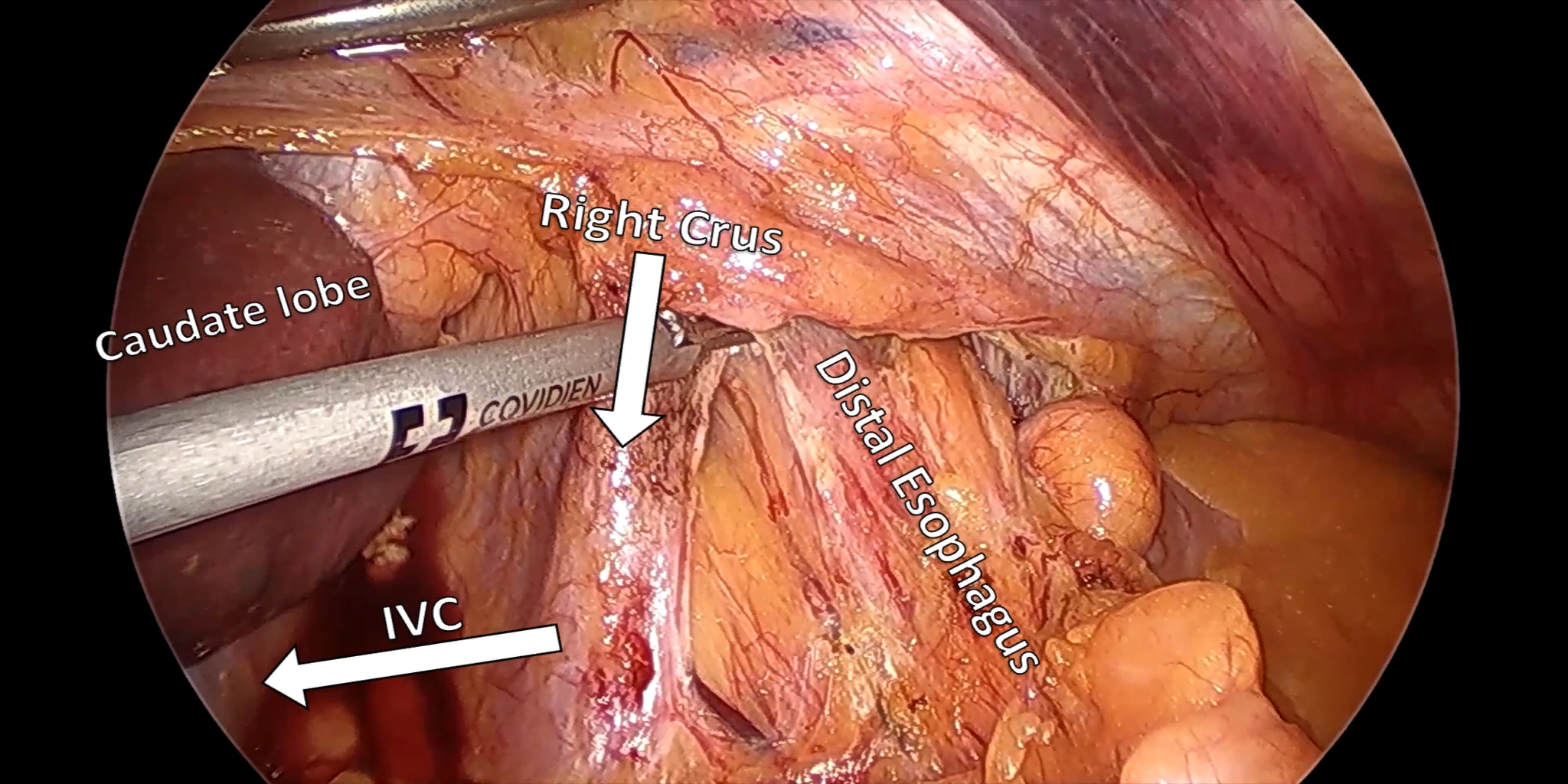
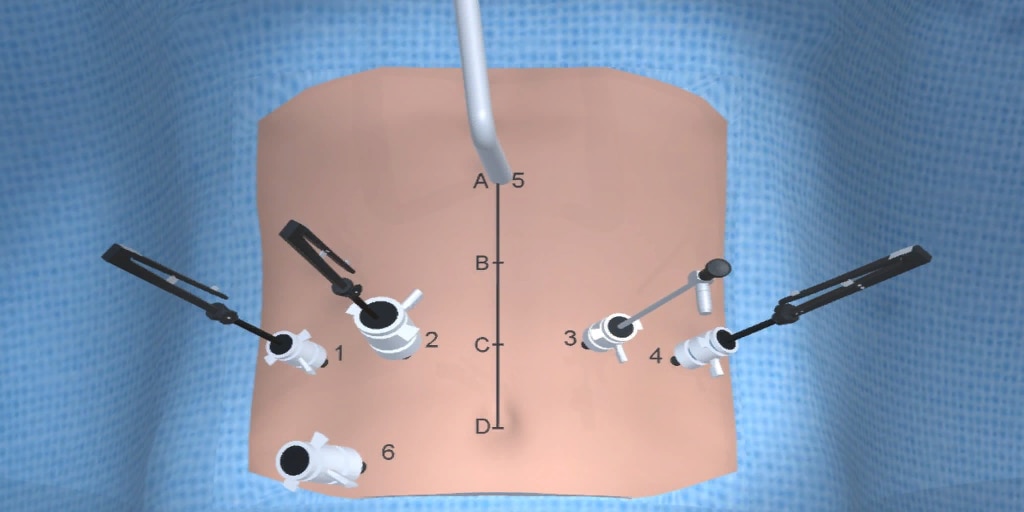
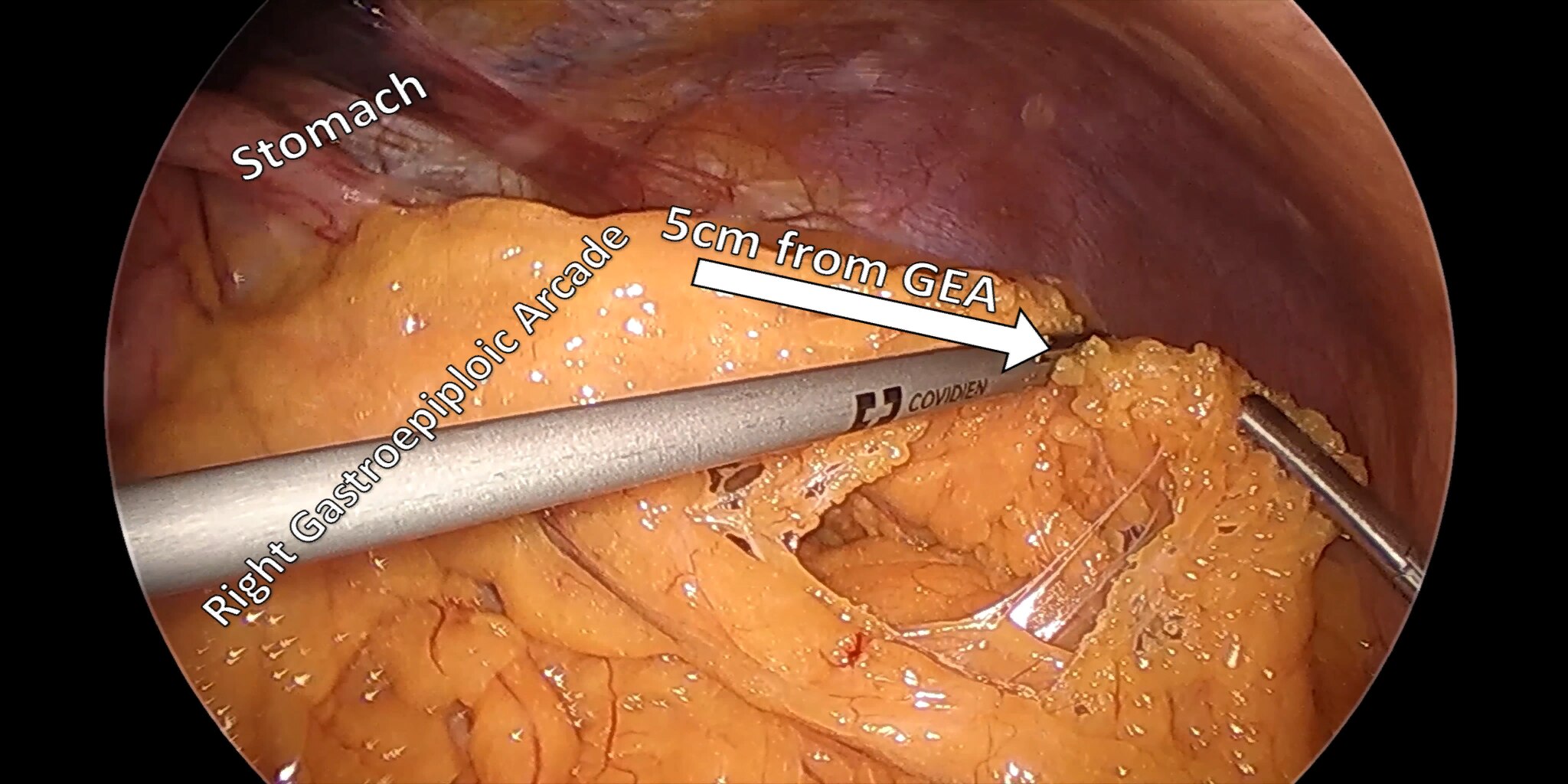
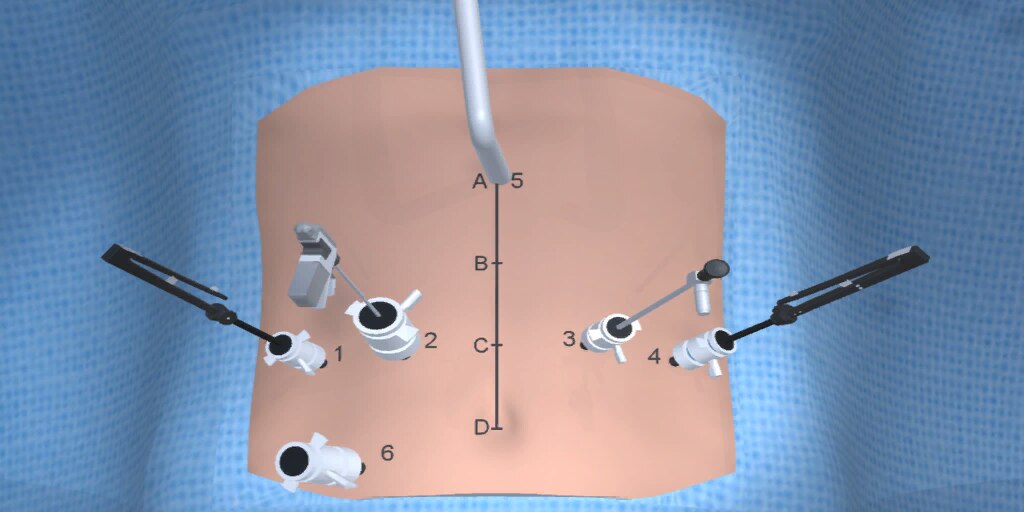
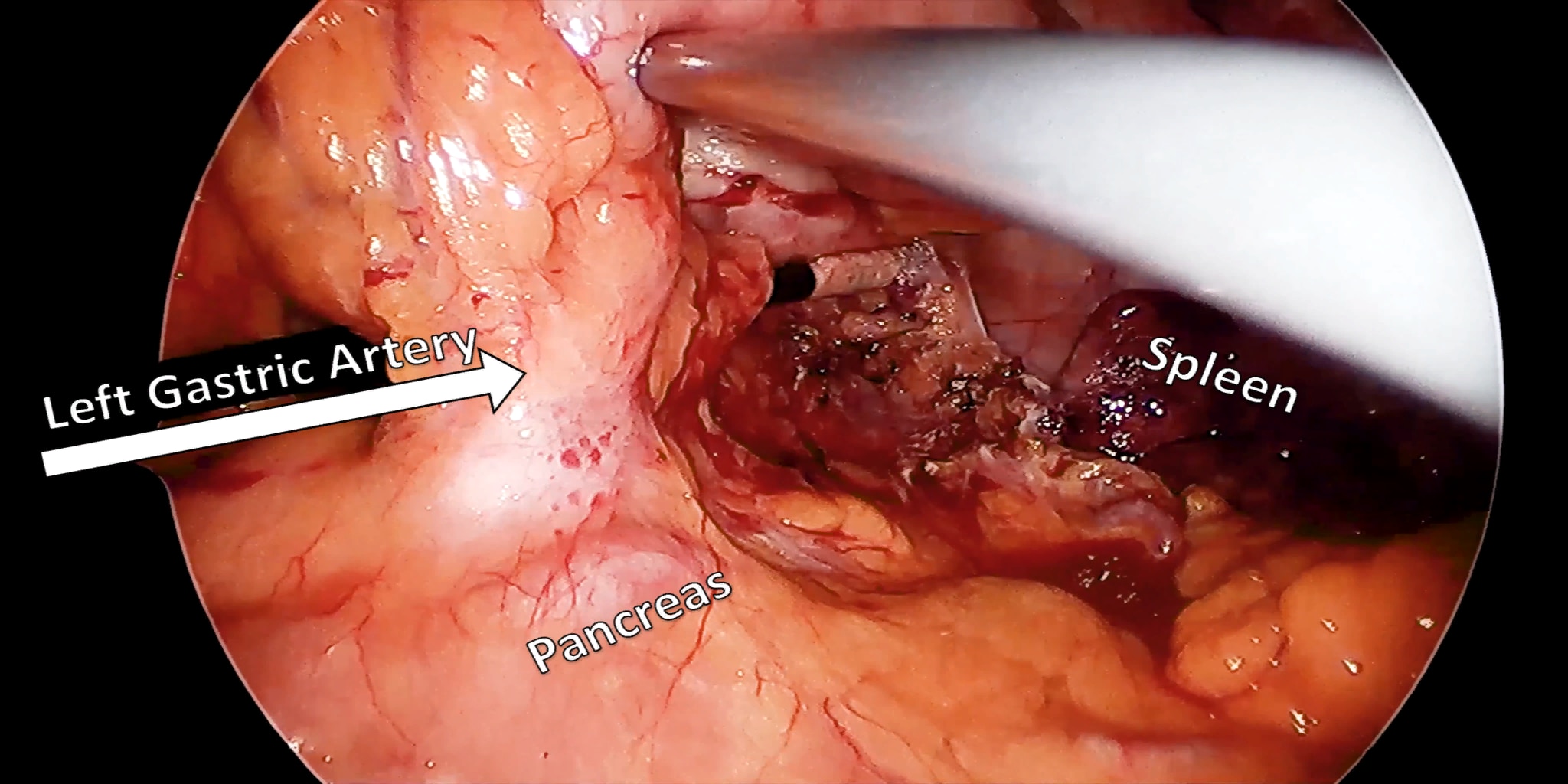
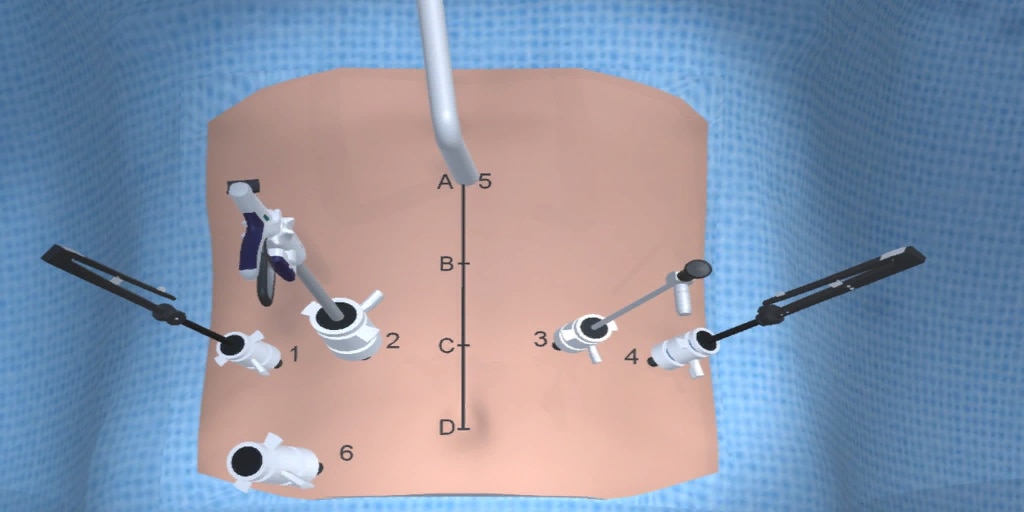
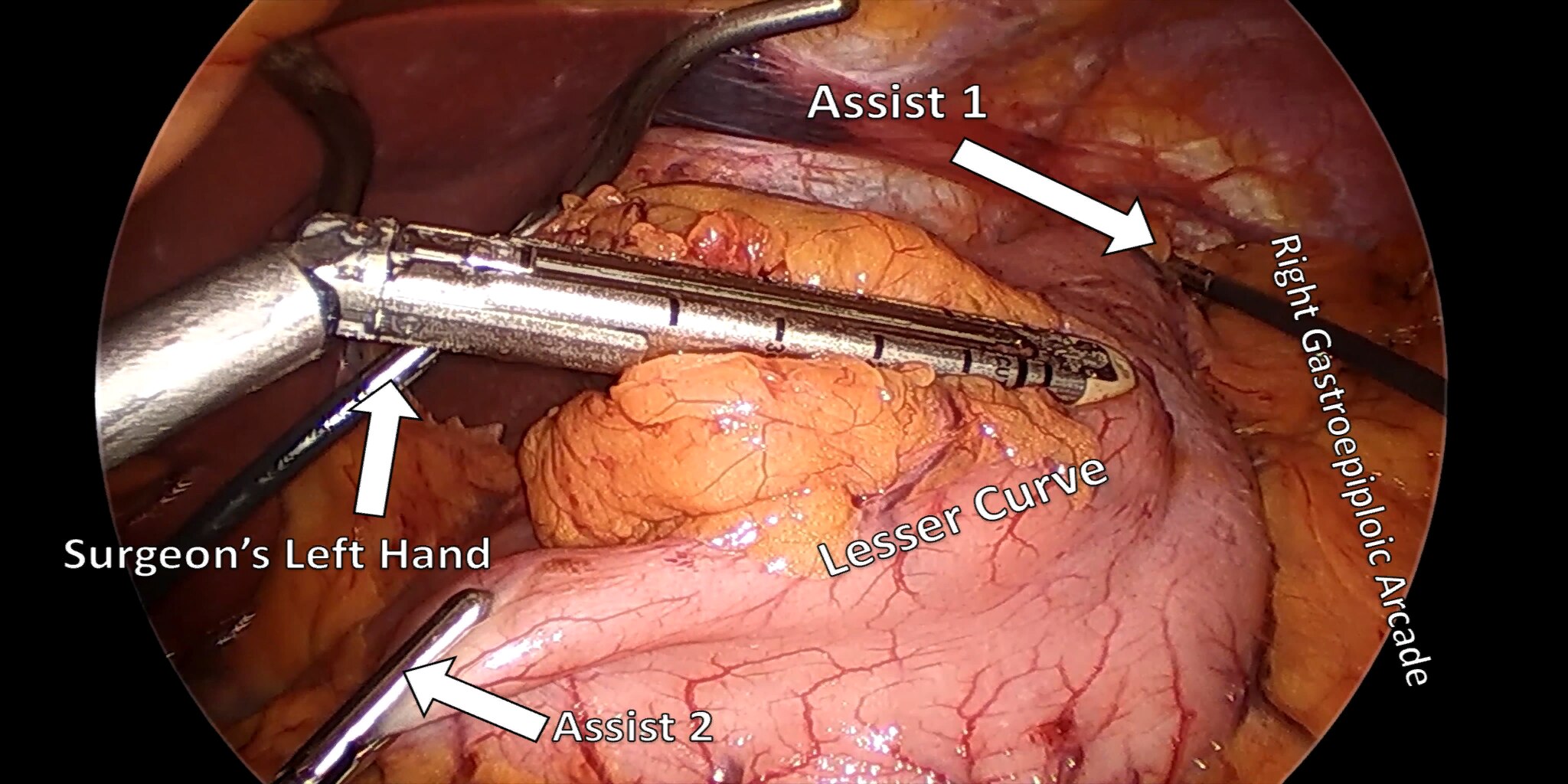
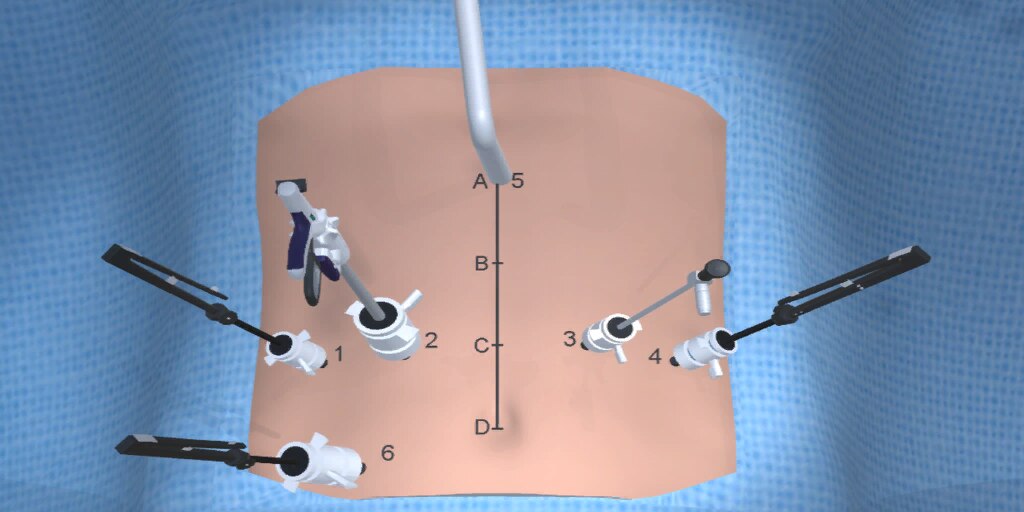
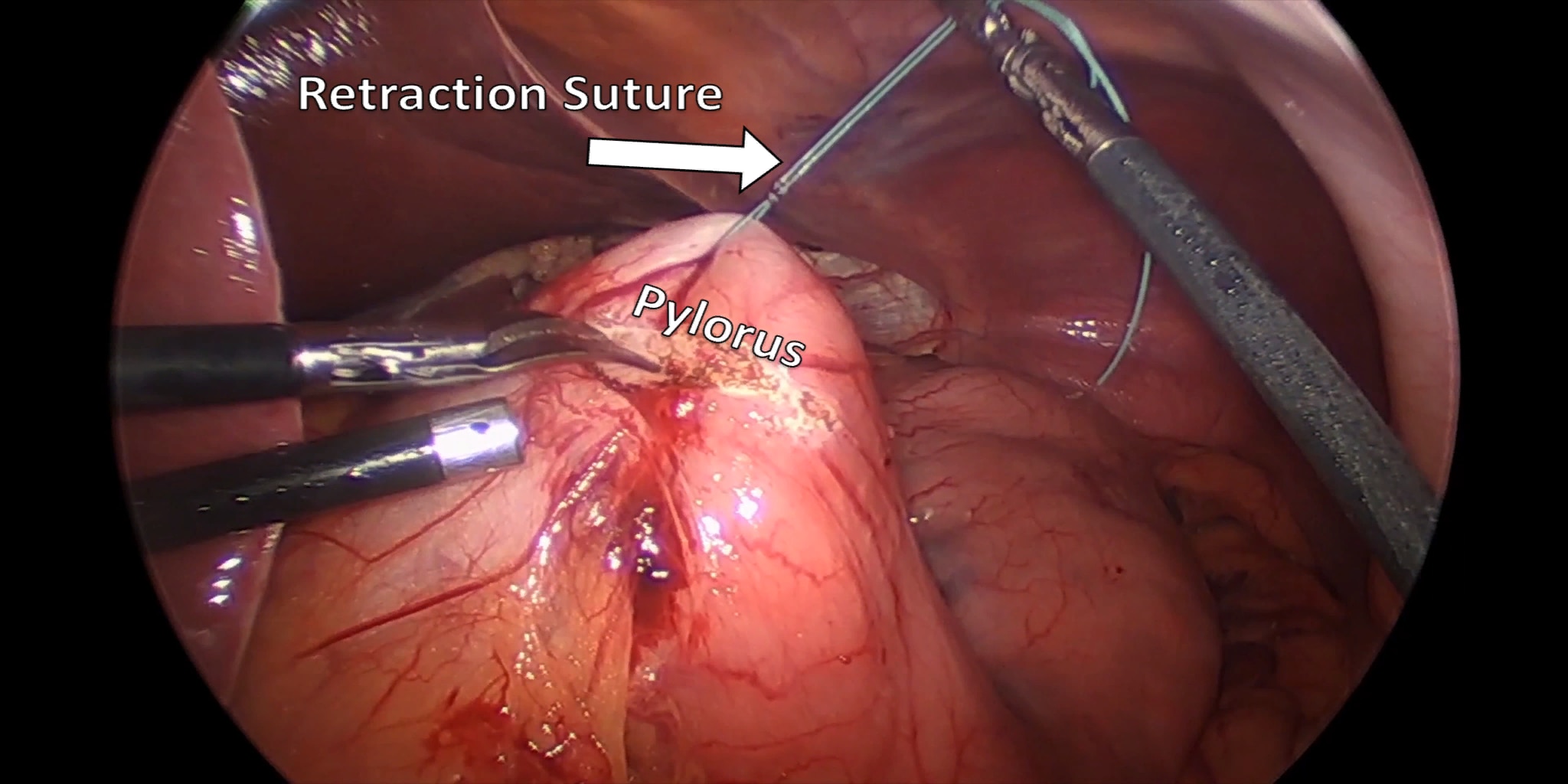
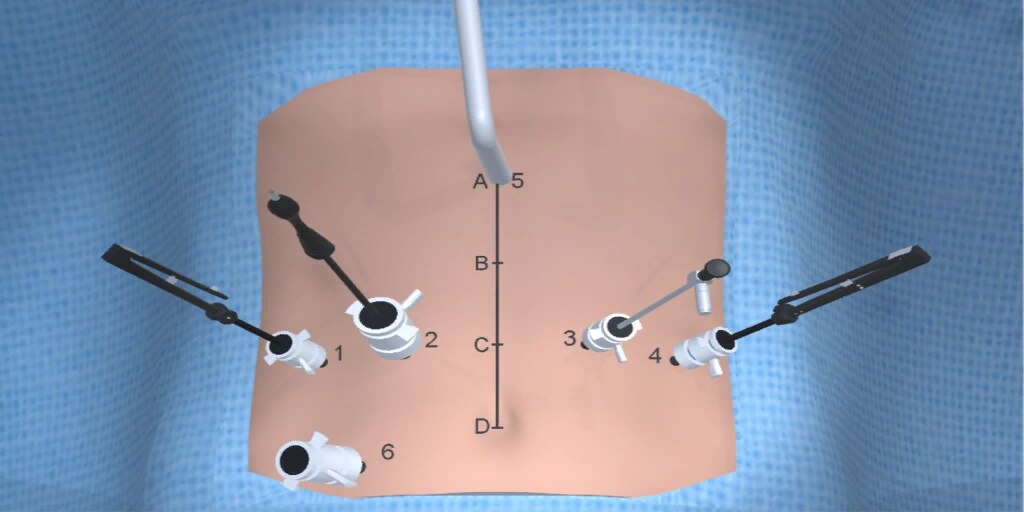
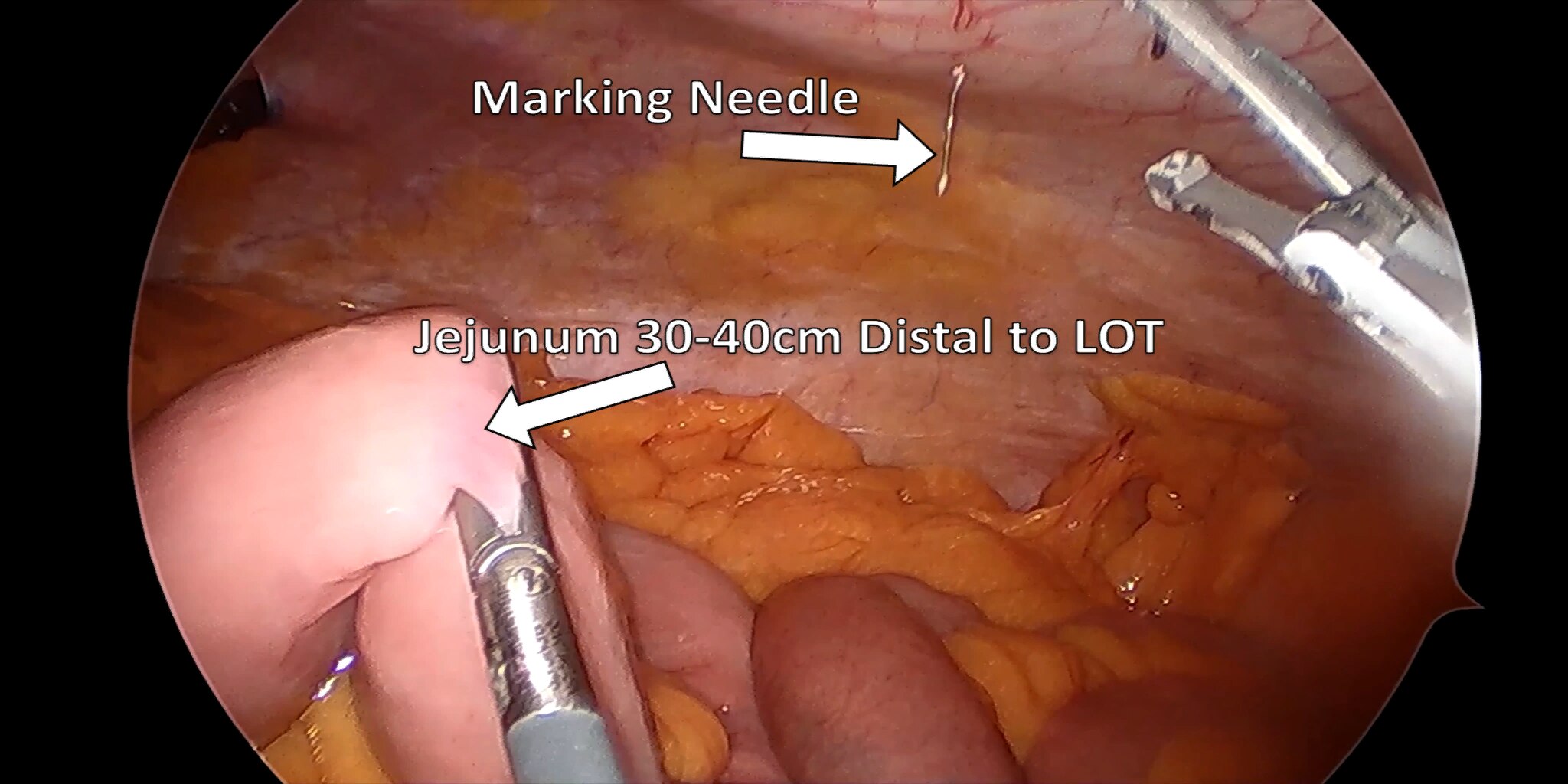
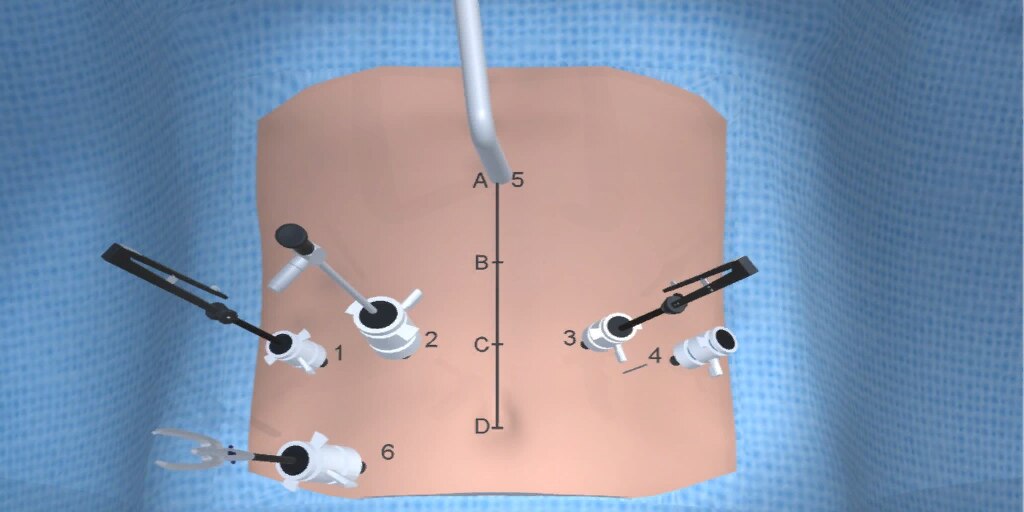
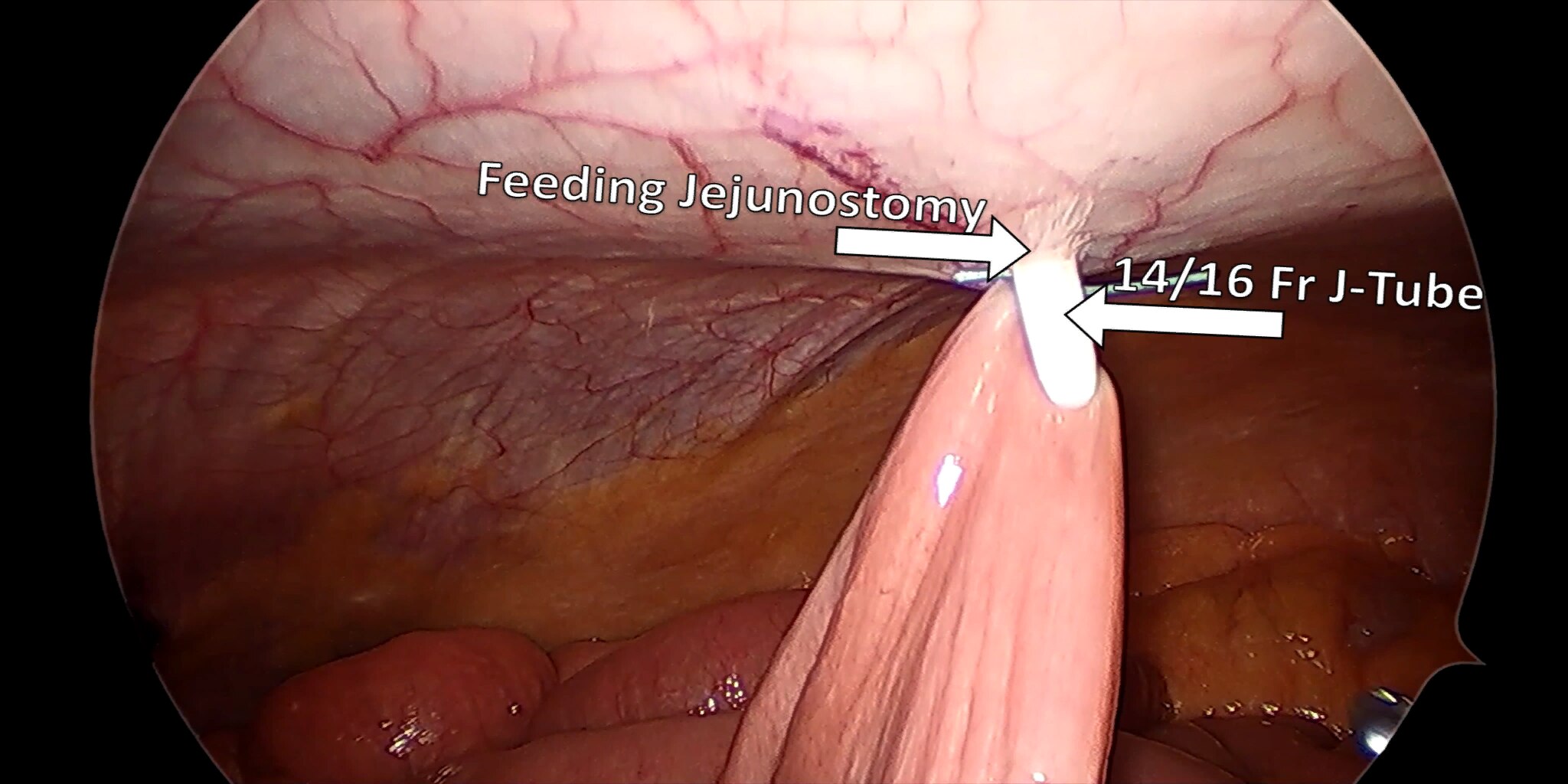
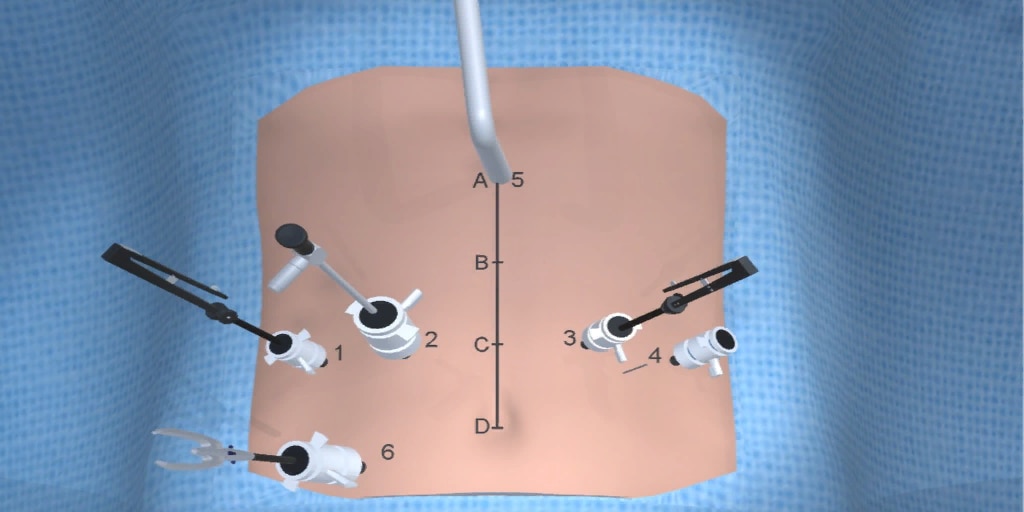
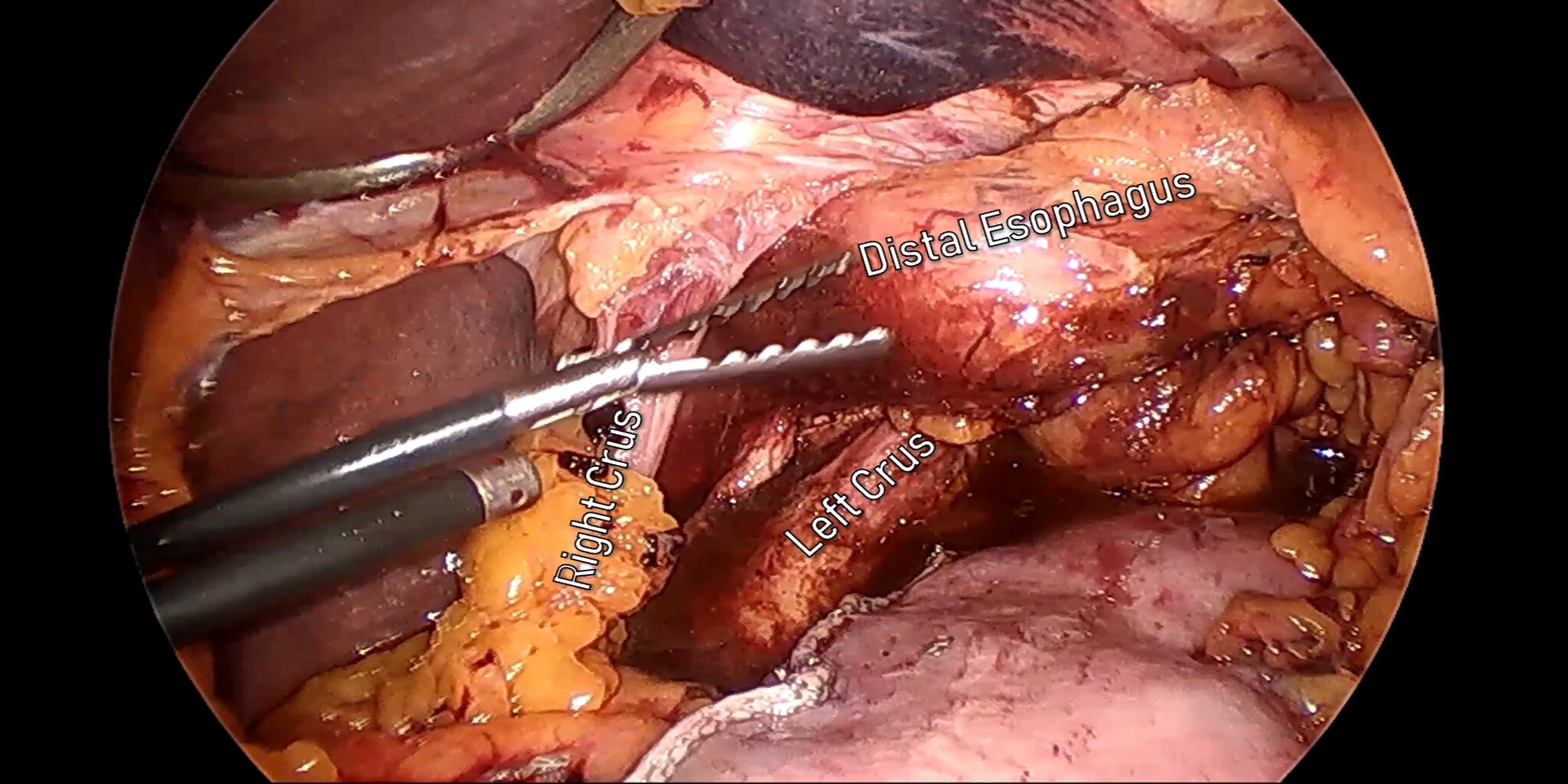
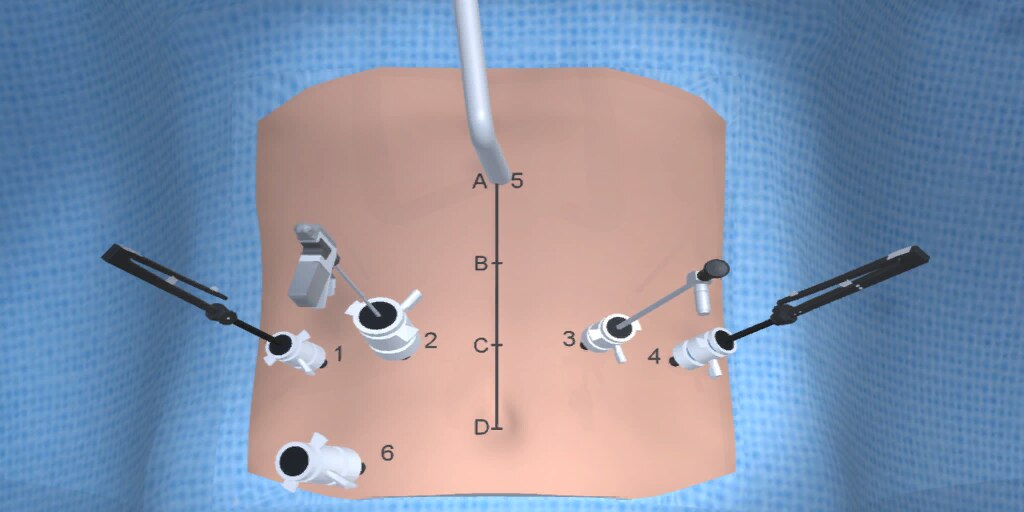
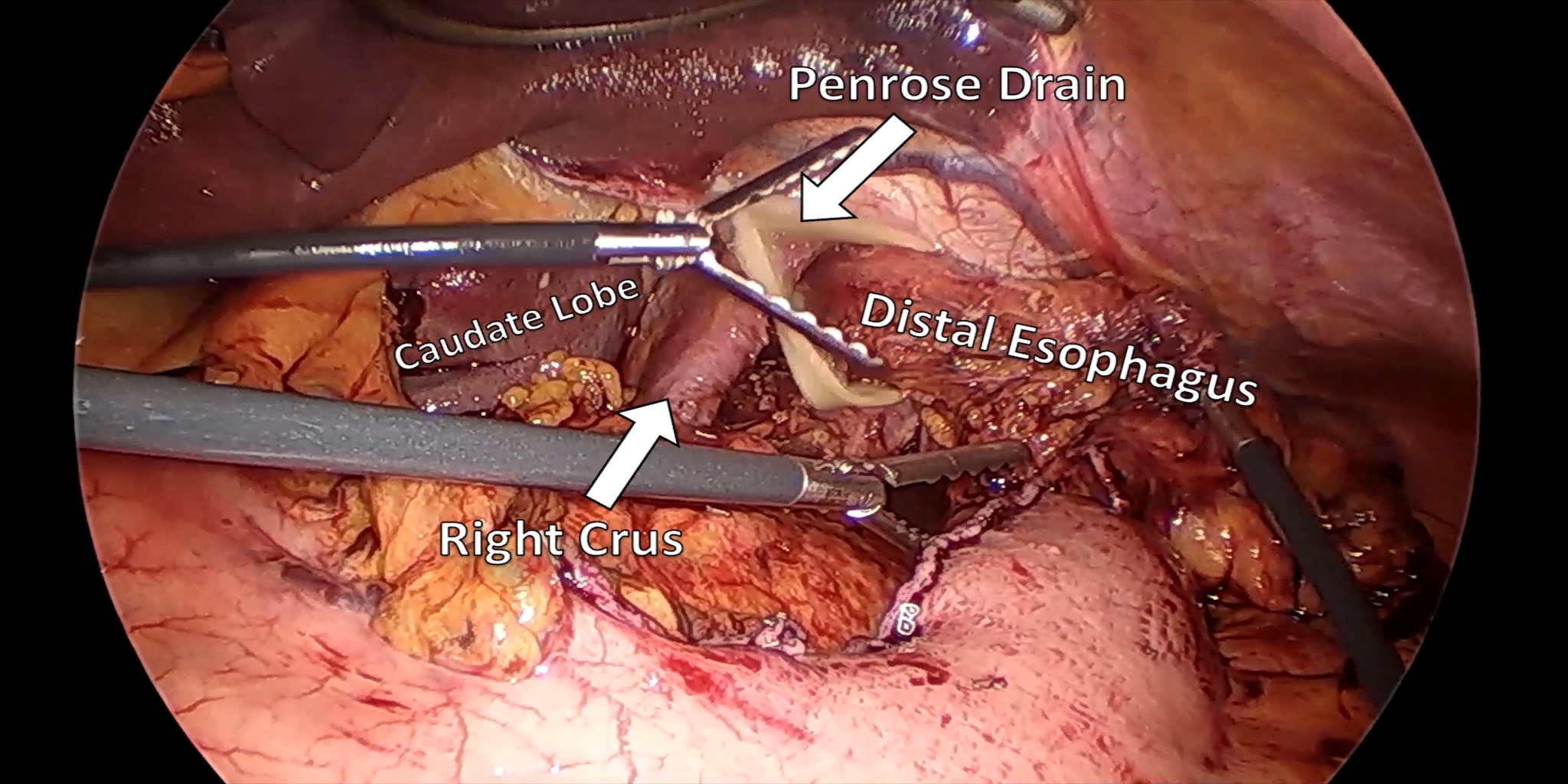
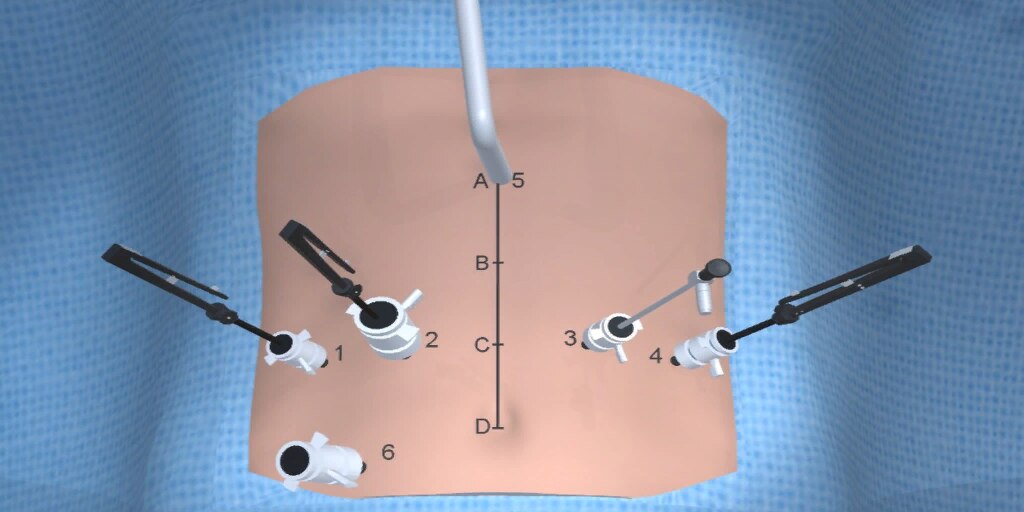
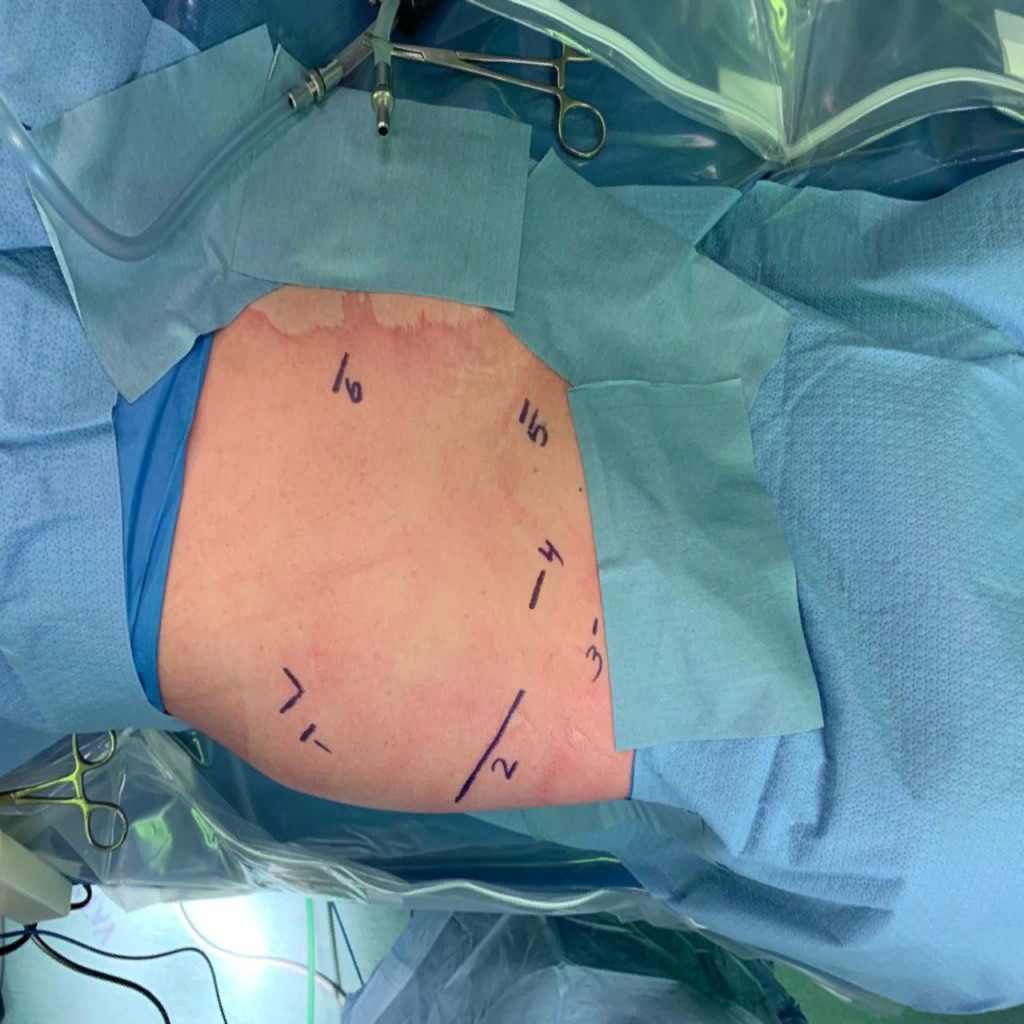
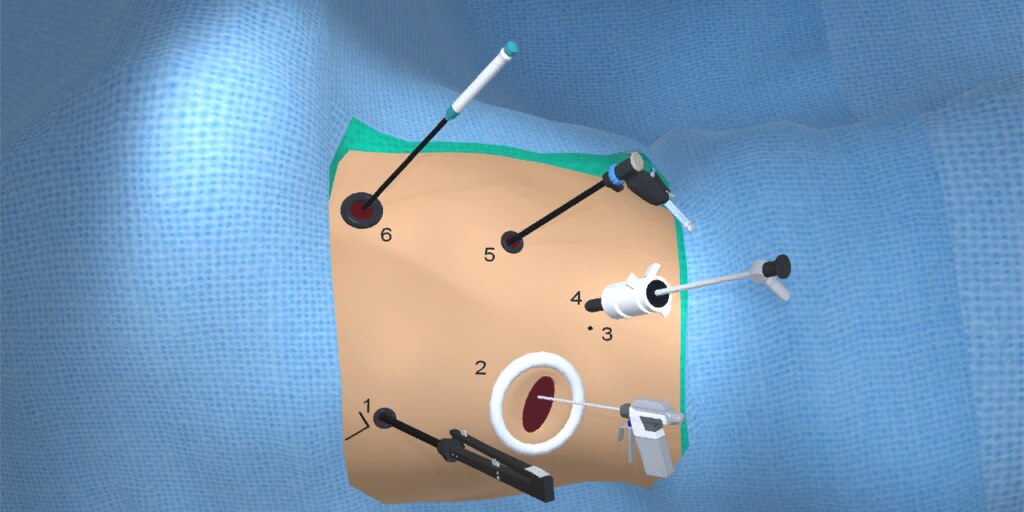
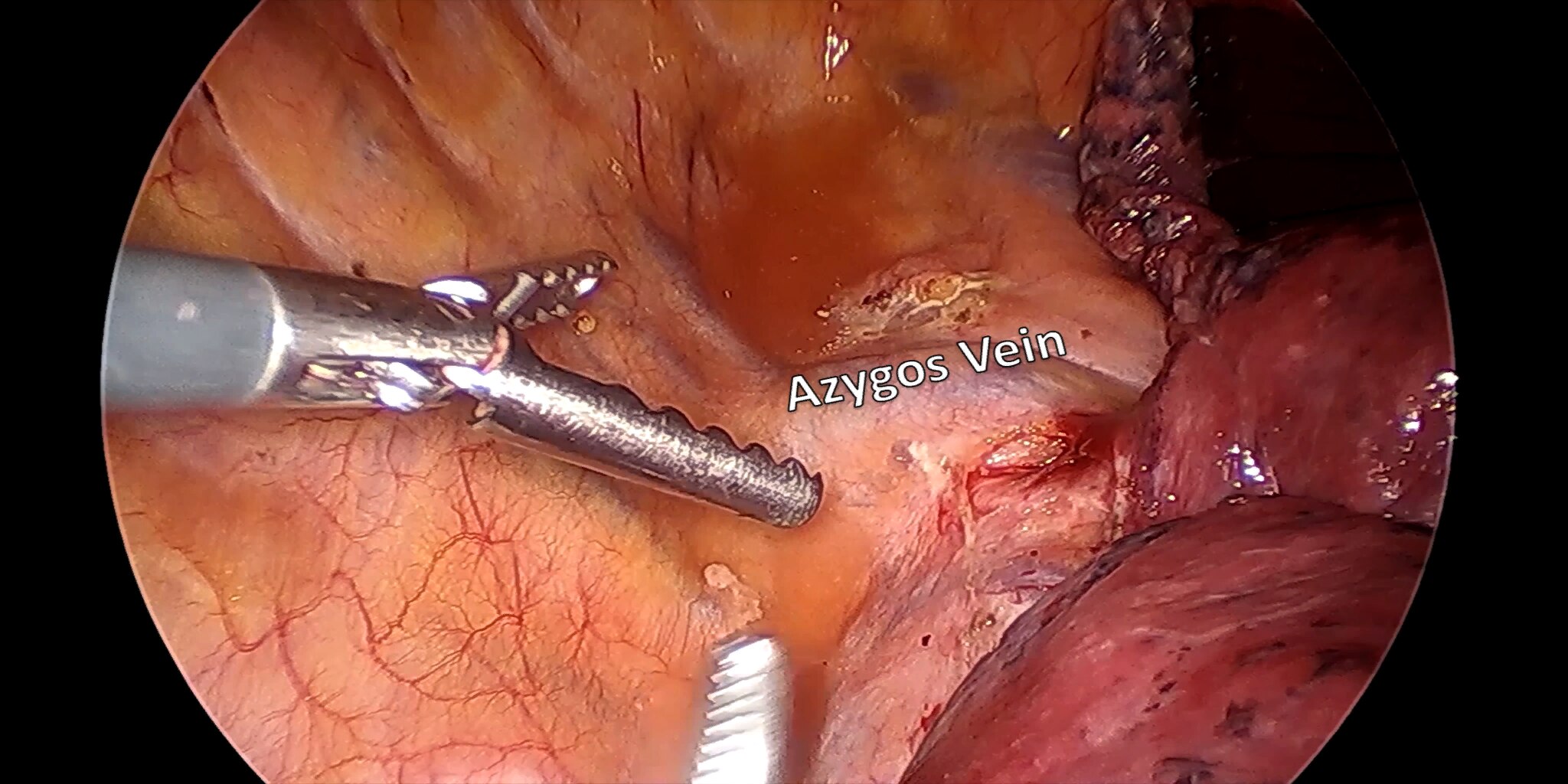
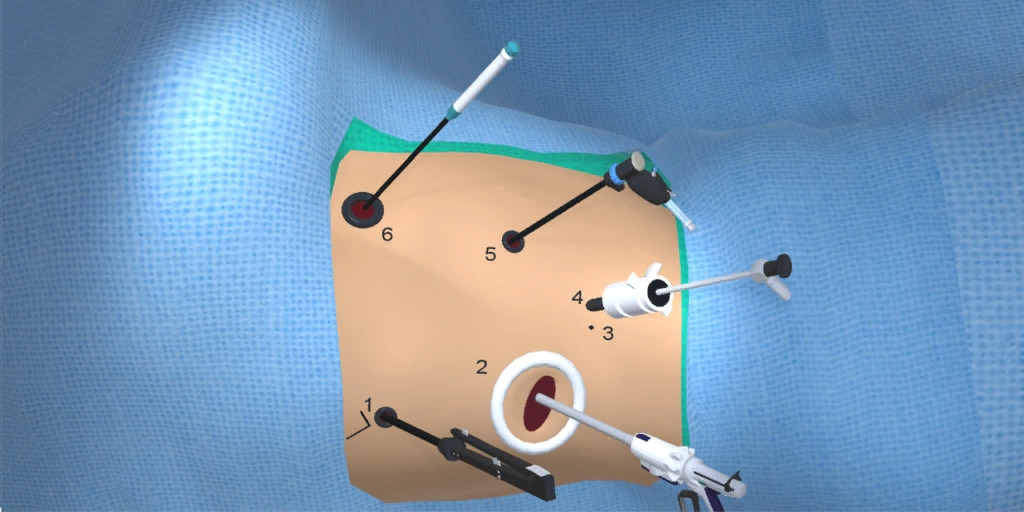
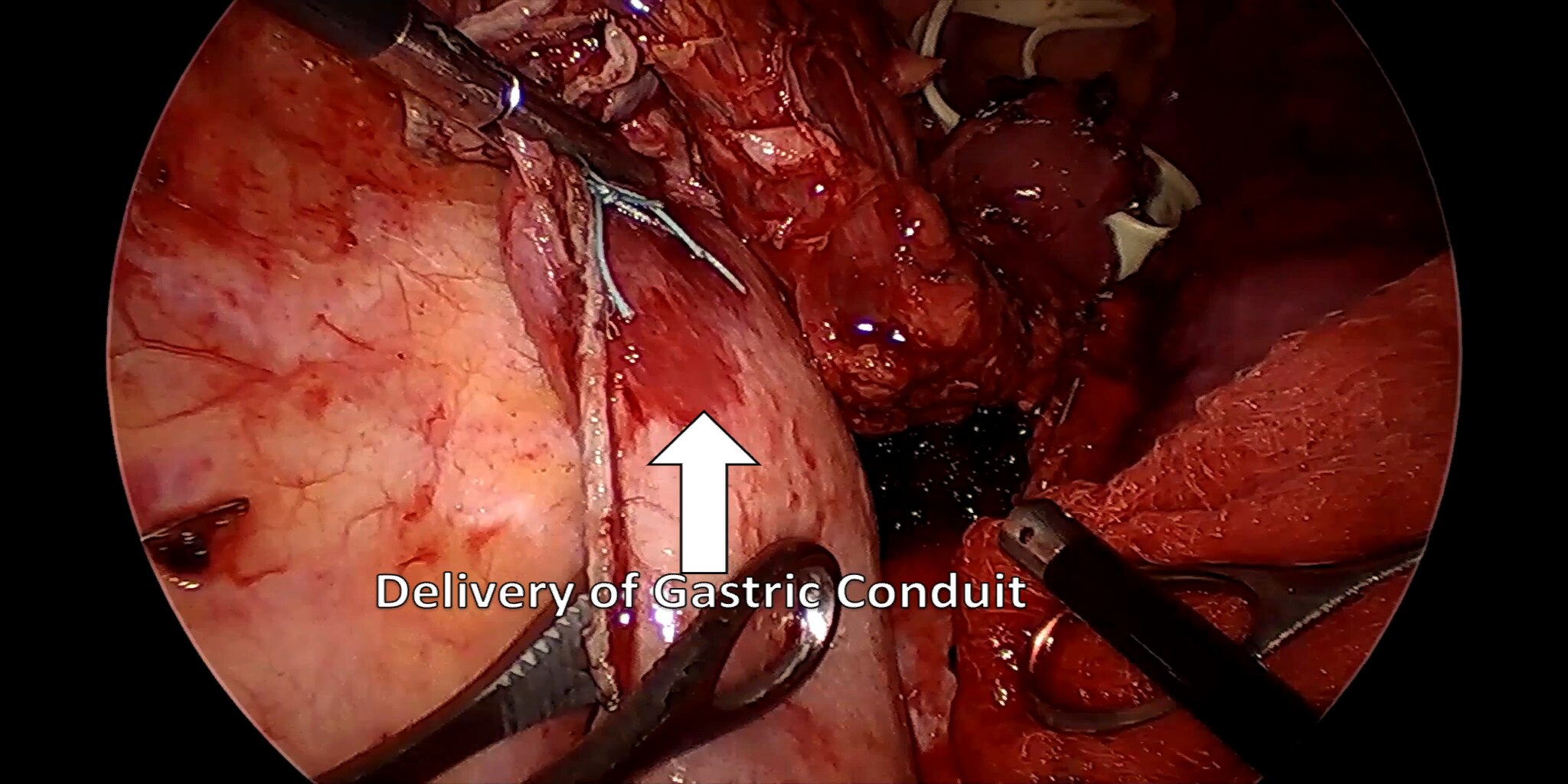
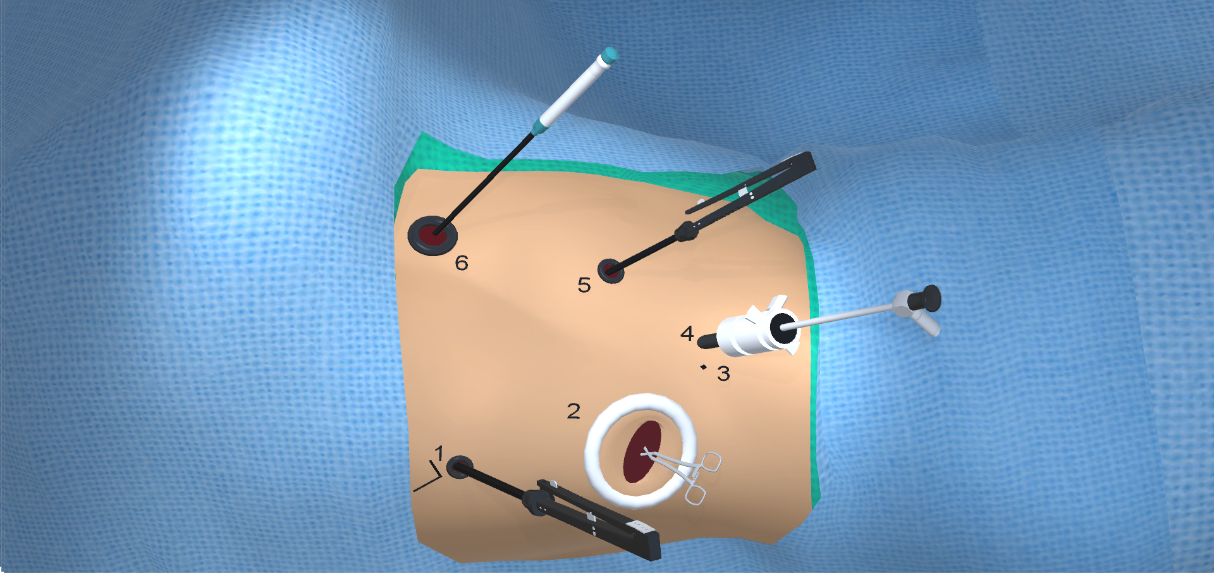
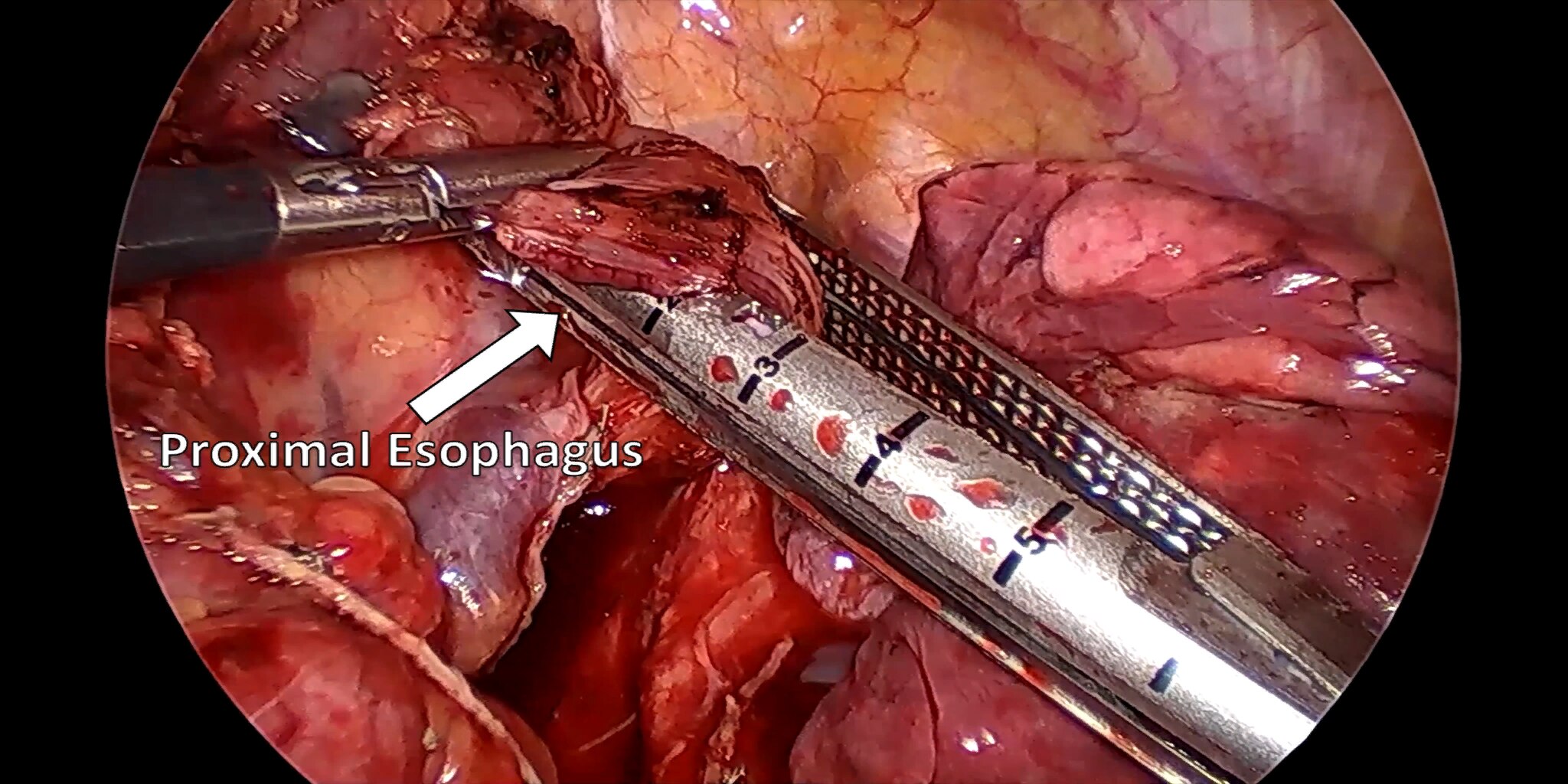
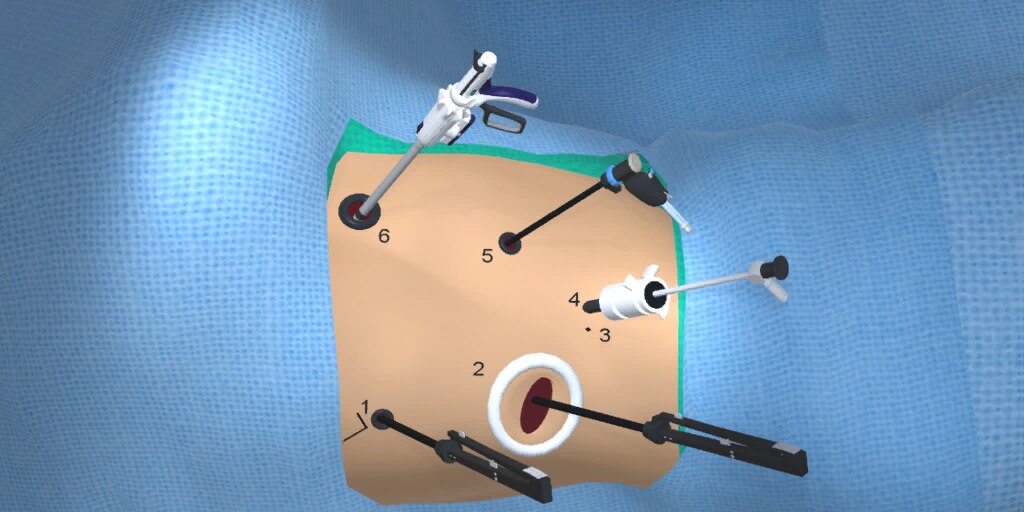
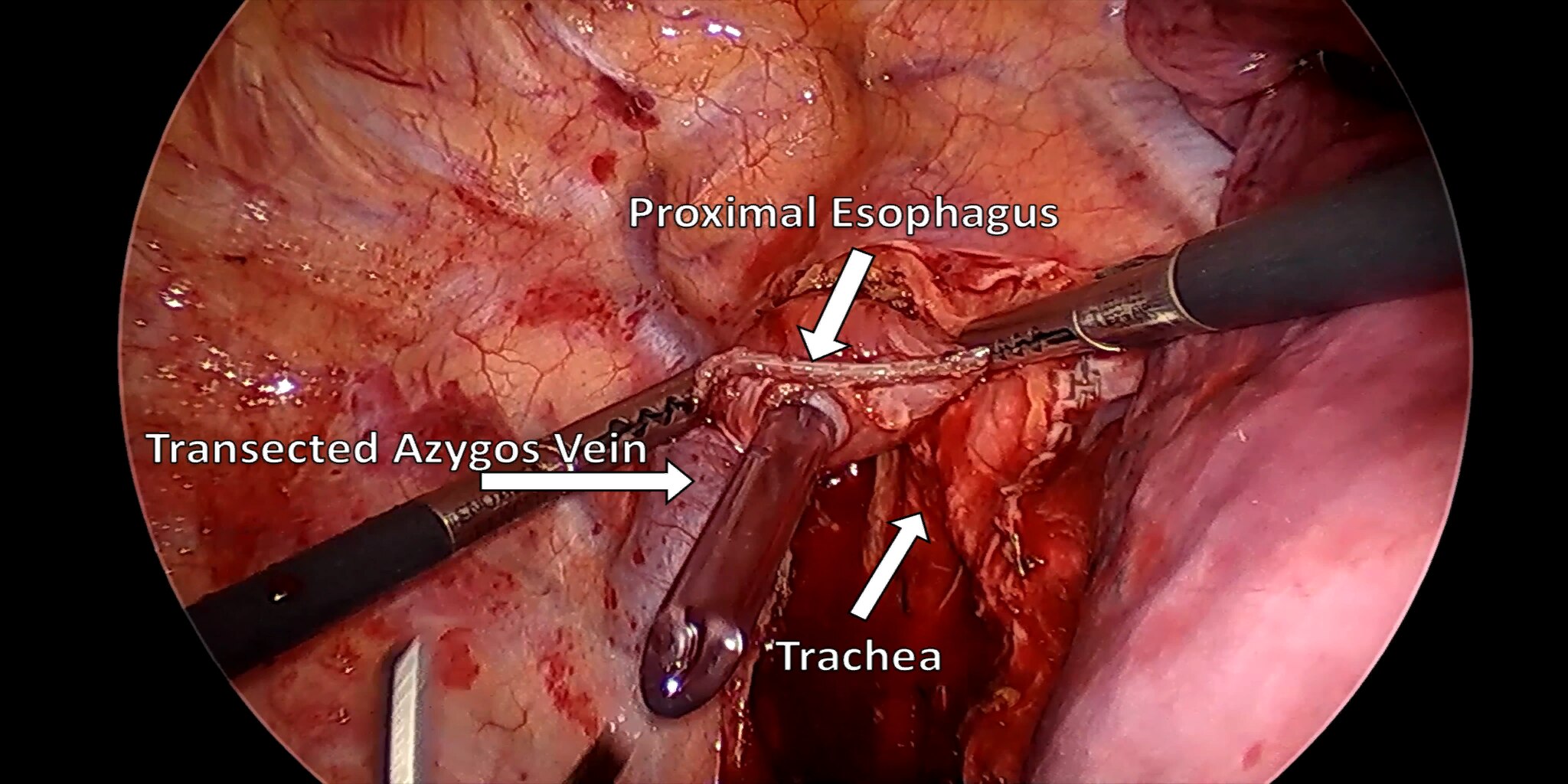
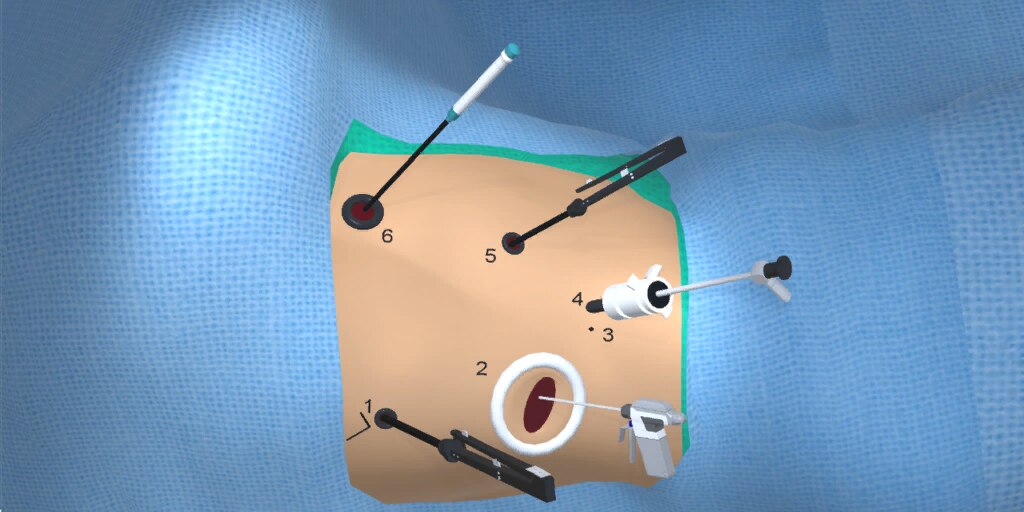
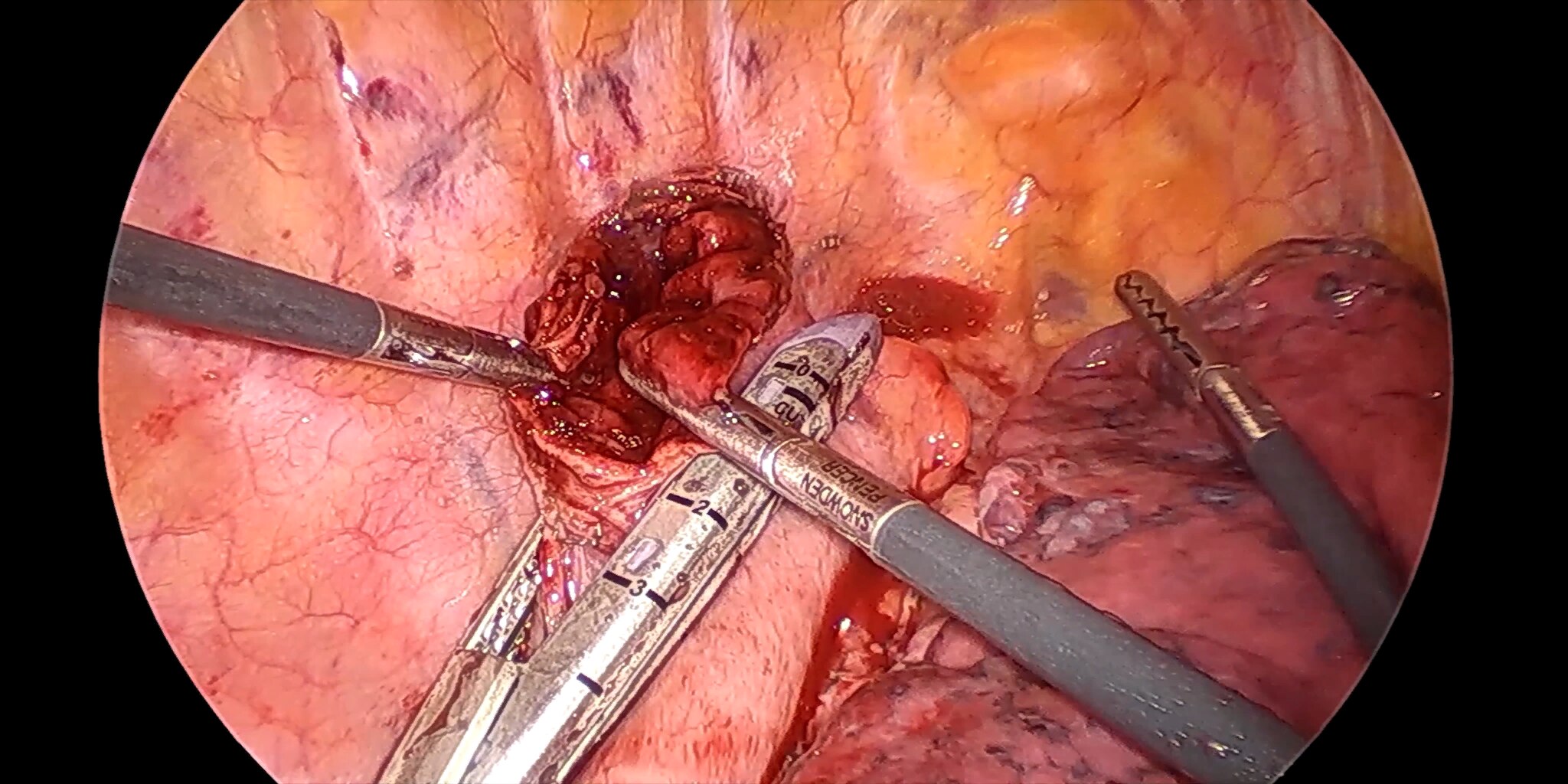
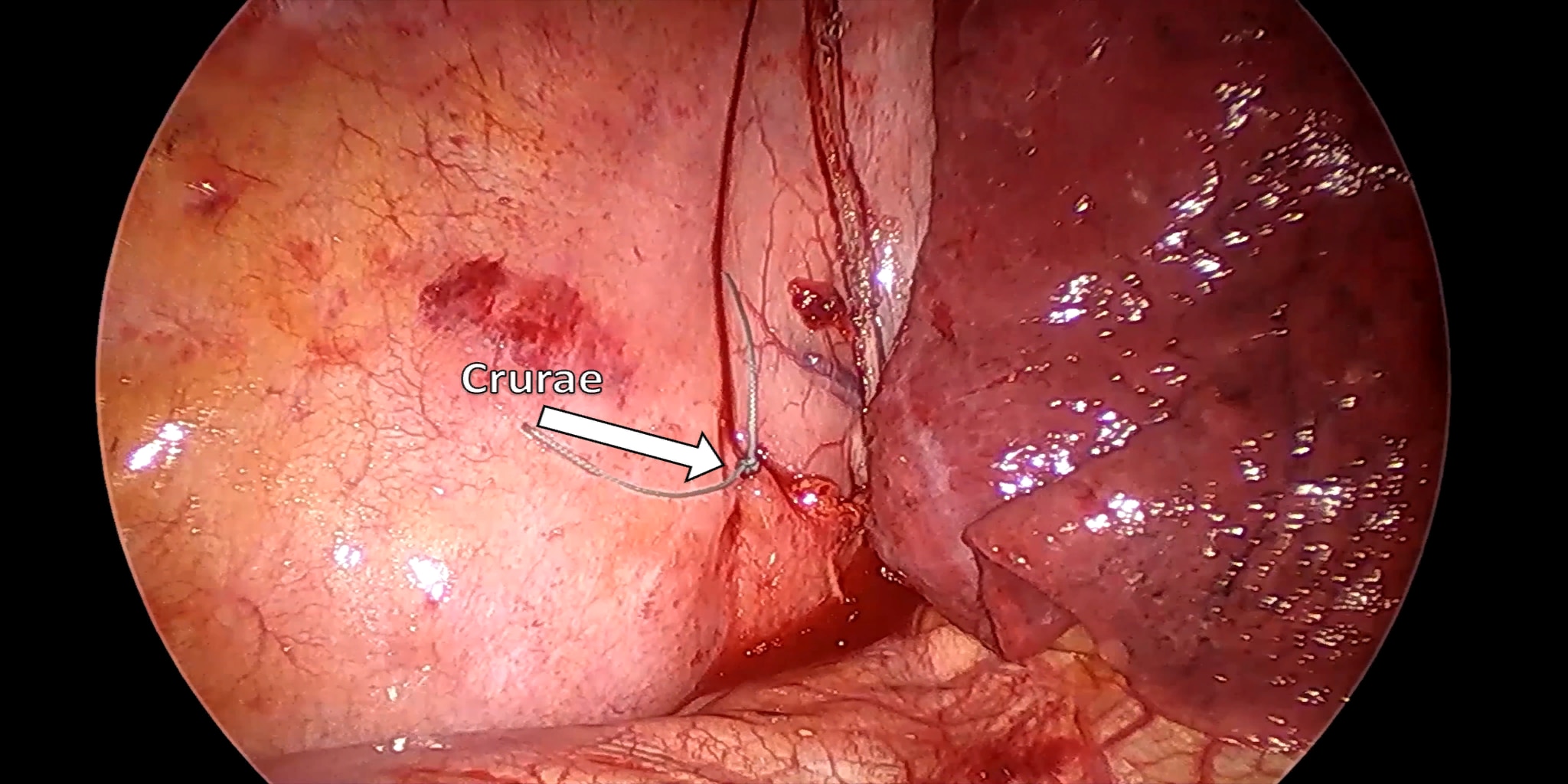
At our center, all patients undergo an esophagogastroduodenoscopy (EGD) to confirm the tumor’s location and the suitability of the stomach as a conduit for reconstruction. The EGD is typically performed a few days preoperatively (sometimes combined with laparoscopic staging, which is performed as an ambulatory procedure), or at the beginning of the operation. If the latter approach is taken, it is important to be mindful of the degree of endoscopic air insufflation, as the air in the small intestines can impair laparoscopic visualization. The operation is divided into two phases: Laparoscopic and Thoracoscopic.